 | |
| Names | |
|---|---|
| IUPAC name
3,3′,3′′,3′′′-[3,8,13,18-Tetrakis(carboxymethyl)-10,15,20,22,23,24-hexahydro-5H,21H-porphyrin-2,7,12,17-tetrayl]tetrapropanoic acid | |
| Systematic IUPAC name
3,3′,3′′,3′′′-[14,33,53,73-Tetrakis(carboxymethyl)-11H,31H,51H,71H-1,3,5,7(2,5)-tetrapyrrolacyclooctaphane-13,34,54,74-tetrayl]tetrapropanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C40H44N4O16 | |
| Molar mass | 836.804 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uroporphyrinogen I is an isomer of uroporphyrinogen III, a metabolic intermediate in the biosynthesis of heme. A type of porphyria is caused by production of uroporphyrinogen I instead of III.
Biosynthesis and metabolism
In living organisms, uroporphyrinogen I occurs as a side branch of the main porphyrin synthesis pathway. In the normal pathway, the linear tetrapyrrole precursor preuroporphyrinogen (a substituted hydroxymethylbilane) is converted by the enzyme uroporphyrinogen-III cosynthase into the cyclic uroporphyrinogen III; which is then converted to coproporphyrinogen III on the way to porphyrins like heme. Uroporphyrinogen I is instead produced spontaneously from preuroporphyrinogen when the enzyme is not present.[1][2]
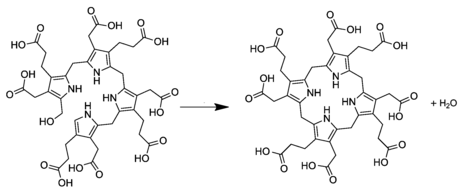
The difference between the I and III forms is the arrangement of the four carboxyethyl groups (propionic acid, "P") and the four carboxymethyl groups (acetic acid, "A"). The non-enzymatic conversion to uroporphyrinogen I results in the sequence AP-AP-AP-AP, whereas the enzymatic conversion into uroporphyrinogen III leads to reversal of one AP-group and hence an AP-AP-AP-PA arrangement.
If synthesized, uroporphyrinogen I is then converted into coproporphyrinogen I by the same enzyme (uroporphyrinogen decarboxylase) that acts on the III form; but that product, which is cytotoxic, then accumulates causing the pathology congenital erythropoietic porphyria.[2][3]
References
- ↑ Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. pp. 1–10. doi:10.1002/9780470048672.wecb221. ISBN 978-0470048672.
- 1 2 Sassa, S.; Kappas, A. (2000). "Molecular aspects of the inherited porphyrias". Journal of Internal Medicine. 247 (2): 169–178. doi:10.1046/j.1365-2796.2000.00618.x. PMID 10692079. S2CID 36820694.
- ↑ Di Pierro, Elena; Brancaleoni, Valentina; Granata, Francesca (2016). "Advances in understanding the pathogenesis of congenital erythropoietic porphyria". British Journal of Haematology. 173 (3): 365–379. doi:10.1111/bjh.13978. PMID 26969896. S2CID 46873299.