 | |
| Names | |
|---|---|
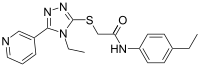
| Preferred IUPAC name
N-(4-Ethylphenyl)-2-{[4-ethyl-5-(pyridin-3-yl)-4H-1,2,4-triazol-3-yl]sulfanyl}acetamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C19H21N5OS | |
| Molar mass | 367.47 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
VUAA1 is a chemical compound that works by over activating an insect's olfactory senses causing a repellent effect. It is considered to be an Orco allosteric agonist.[1] It was discovered at Vanderbilt University[2] with research being partially funded by the Bill and Melinda Gates Foundation.[3]
VUAA1 is an agonist believed to work by overloading an insect's odorant receptors. It may be 1000 times stronger than DEET[4] and may lead to, "a powerful new family of compounds that can be used to disrupt the destructive behaviors of nuisance insects, agricultural pests, and disease vectors alike."[5]
VUAA1 has also been shown to stimulate mosquito sperm motility, thus showing a link between a mosquito's sense of smell and reproduction.[6]
References
- ↑ Medicine, Vanderbilt University School of. "Allosteric antagonism of insect odorant receptor ion channels. | Chemical Synthesis Core". medschool.vanderbilt.edu. Retrieved 2016-06-02.
- ↑ Jones, P. L.; Pask, G. M.; Rinker, D. C.; Zwiebel, L. J. (2011). "Functional agonism of insect odorant receptor ion channels". Proceedings of the National Academy of Sciences. 108 (21): 8821–5. Bibcode:2011PNAS..108.8821J. doi:10.1073/pnas.1102425108. PMC 3102409. PMID 21555561.
- ↑ Doug Gross (5 May 2014). "A bug repellent that could save lives - CNN.com". CNN. Retrieved 2016-06-02.
- ↑ "New type of insect repellent may be thousands of times stronger than DEET" (Press release). physorg.com. May 9, 2011.
- ↑ "Functional agonism of insect odorant receptor ion channel" (Press release). April 4, 2011.
- ↑ "VICB Article: Sulfilimine Cross-Links A Key to Tissue Evolution". www.vanderbilt.edu. Retrieved 2016-06-02.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.