 | |
| Clinical data | |
|---|---|
| Trade names | Ezharmia |
| Other names | Valemetostat tosilate (JAN); DS-3201; DS-3201b |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
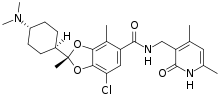
| Formula | C26H34ClN3O4 |
| Molar mass | 488.03 g·mol−1 |
Valemetostat (trade name Ezharmia) is a drug used for the treatment of adult T-cell leukemia/lymphoma (ATL).
Valemetostat is an inhibitor of the enzymes enhancer of zeste homolog 1 (EZH1) and enhancer of zeste homolog 2 (EZH2), which are implicated in the etiology of some forms of cancer including non-Hodgkin lymphomas.[1][2]
In Japan, valemetostat was approved in September 2022 for patients with relapsed or refractory adult T-cell leukemia/lymphoma.[3][4]
References
- ↑ Ishitsuka K, Izutsu K, Maruyama D, Makita S, Jacobsen ED, Horwitz S, et al. (2021). "First‐In‐Human Study of the Ezh1 and Ezh2 Dual Inhibitor Valemetostat Tosylate (Ds‐3201B) in Patients with Relapsed or Refractory Non‐Hodgkin Lymphomas". Hematological Oncology. 39. doi:10.1002/hon.14_2879. S2CID 237853028.
- ↑ Morishima S, Ishitsuka K, Izutsu K, Kusumoto S, Makiyama J, Utsunomiya A, et al. (2019). "First-in-Human Study of the EZH1/2 Dual Inhibitor Valemetostat in Relapsed or Refractory Non-Hodgkin Lymphoma (NHL) - Updated Results Focusing on Adult T-Cell Leukemia-Lymphoma (ATL)". Blood. 134: 4025. doi:10.1182/blood-2019-125507. S2CID 209291086.
- ↑ Dou F, Tian Z, Yang X, Li J, Wang R, Gao J (October 2022). "Valemetostat: First approval as a dual inhibitor of EZH1/2 to treat adult T-cell leukemia/lymphoma". Drug Discoveries & Therapeutics. doi:10.5582/ddt.2022.01085. PMID 36310058. S2CID 253189013.
- ↑ "Japan Green Lights Valemetostat Tosilate for Relapsed/Refractory Adult T-cell Leukemia/Lymphoma". OncLive. September 26, 2022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.