| WWE protein domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
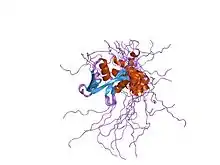 Solution structure of WWE domain in BAB28015 | |||||||||
| Identifiers | |||||||||
| Symbol | WWE | ||||||||
| Pfam | PF02825 | ||||||||
| InterPro | IPR004170 | ||||||||
| |||||||||
Function
The WWE domains occur in two functional classes of proteins, namely those associated with ubiquitination and those associated with poly-ADP ribosylation (PARP). Hence, WWE domains hold an important function in signal transduction, protein degradation, DNA repair and apoptosis.[1]
Protein Interactions
The WWE domain is named after three of its conserved residues, W and E residues (tryptophans and glutamate respectively), and is predicted to mediate specific protein-protein interactions in ubiquitin and ADP ribose conjugation systems (Poly ADP ribose polymerase). This domain is found as a tandem repeat at the N-terminal of Deltex, a cytosolic effector of Notch signalling thought to bind the N-terminal of the Notch receptor.[2] It is also found as an interaction module in protein ubiquination and ADP ribosylation proteins.[1]
Structure
Within each WWE module, the residues form two similar structures vital to its stability. The two WWE modules interact and form a large cleft suitable for binding of extended polypeptides. The two WWE modules adopt compact structures mostly composed of beta strands, with a single three turn alpha helix in both modules and an additional short helical segment in the second WWE module. The two WWE modules hold a two-fold rotation axis.[2]
References
- 1 2 Aravind L (2001). "The WWE domain: a common interaction module in protein ubiquitination and ADP ribosylation". Trends Biochem Sci. 26 (5): 273–5. doi:10.1016/s0968-0004(01)01787-x. PMID 11343911.
- 1 2 Zweifel ME, Leahy DJ, Barrick D (November 2005). "Structure and Notch receptor binding of the tandem WWE domain of Deltex". Structure. 13 (11): 1599–611. doi:10.1016/j.str.2005.07.015. PMID 16271883.