 | |
| Names | |
|---|---|
| IUPAC name
[(2S,4R)-2,3,4,5-tetraacetyloxypentyl] acetate | |
| Other names
[(2S,4R)-2,3,4,5-Tetraacetyloxypentyl] acetate D-Ribitol pentaacetate 1,2,3,4,5-penta-O-acetyl ribitol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C15H22O10 | |
| Molar mass | 362.331 g·mol−1 |
| Soluble in water[1] | |
| Solubility | Soluble in methanol [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
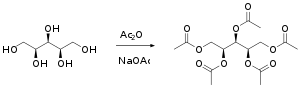
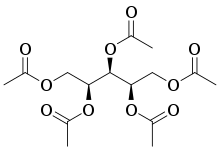
Xylitol pentacetate is an organic compound with the formula C15H22O10. It is an acetylated sugar alcohol that is used as an intermediary in the production of xylitol pentanitrate.[2] It is also commonly made to isolate and identify xylitol from complex organic mixtures.[3][1]
Synthesis
Xylitol pentacetate is made by the addition of acetic anhydride and sodium acetate to xylitol.[1]
References
- 1 2 3 4 Hockett, R. C.; Hudson, C. S. (1935). "Emil Fischer's Assignment of Configuration to d-Glucose. The Pentaacetates of d-Arabitol and d-Xylitol". Journal of the American Chemical Society. 57 (9): 1753. doi:10.1021/ja01312a502. ISSN 0002-7863.
- ↑ Wright, I. G.; Hayward, L. D. (February 1960). "The Pentitol Pentanitrates". Canadian Journal of Chemistry. 38 (2): 316–319. doi:10.1139/v60-045. ISSN 0008-4042.
- ↑ Moers, M.E.C.; Jones, D.M.; Eakin, P.A.; Fallick, A.E.; Griffiths, H.; Larter, S.R. (1993). "Carbohydrate diagenesis in hypersaline environments: application of GC-IRMS to the stable isotope analysis of derivatized saccharides from surficial and buried sediments". Organic Geochemistry. 20 (7): 927–933. doi:10.1016/0146-6380(93)90104-J.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.