 | |
| Names | |
|---|---|
| Other names
Zirconium(IV) lactate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H20O12Zr | |
| Molar mass | 447.504 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
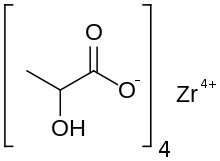
Zirconium lactate is the zirconium salt of lactic acid. It is used in some deodorants. Zirconium carboxylates adopt highly complex structures and are heterogeneous compositions with the approximate formula Zr(OH)4-n(O2CCHOHCH3)n(H2O)x where 1 < n < 3.[1]
Uses
It is also used in the petroleum industry as a cross-linking agent to prepare gels for fracturing fluids, fluids which are pumped into an oil-bearing rock formation to cause cracks in the rock and so to allow the oil to be extracted.[2] It may be prepared by treating zirconium oxide with lactic acid.[2]
Physical properties
It is a colourless solid.
Safety
Its LD50 >10 g/kg).[3] It is suspected of causing zirconium granulomas (a form of skin irritation) in a small number of users.[4]
References
- ↑ Ralph Nielsen "Zirconium and Zirconium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a28_543
- 1 2 Dawson, Jeffrey C.; Le Hoang, Van (31 October 1996), "Gelation Additive for Hydraulic Fracturing Fluids", International Patent Application, WO9633966
- ↑ Brown, J. R.; Mastromatteo, E.; Horwood, J. (1963), "Zirconium lactate and barium zirconate. Acute toxicity and inhalation effects in experimental animals", Am. Ind. Hyg. Assoc. J., 24 (2): 131–366, doi:10.1080/00028896309342940, PMID 14015998
- ↑ James, William D.; Berger, Timothy G.; et al. (2006), Andrews' Diseases of the Skin: Clinical Dermatology, Saunders Elsevier, p. 46, ISBN 0-7216-2921-0
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.