Zooarchaeology by mass spectrometry, commonly referred to by the abbreviation ZooMS, is a scientific method that identifies animal species by means of characteristic peptide sequences in the protein collagen. ZooMS is the most common archaeological application of peptide mass fingerprinting (PMF) and can be used for species identification of bones, teeth, skin and antler. It is commonly used to identify objects that cannot be identified morphologically. In an archaeological context this usually means that the object is too fragmented or that it has been shaped into an artefact. Archaeologists use these species identification to study among others past environments, diet and raw material selection for the production of tools.
Developmental history
ZooMS was first published in 2009[1] by a team of researchers from the University of York, but the term was coined later in a publication in 2010.[2] The original aim of ZooMS was to distinguish between sheep and goat. The bones of these two closely related species are difficult to distinguish, especially when fragmented, yet the difference between these two common domesticates is very important for our understanding of past husbandry practices.
Most of the method development following the initial publication of ZooMS has focused on the extraction of collagen from the archaeological material. In the original protocol acid was used to dissolve the bone’s mineral matrix and free up the collagen. In 2011 an alternative extraction method was published that used an ammonium bicarbonate buffer to solubilise the collagen without dissolving the mineral matrix.[3] In contrast to the acid protocol, the ammonium bicarbonate protocol does not affect the size and mass of the sample, making it a much less destructive method compared to the original protocol. In fact, the ammonium bicarbonate protocol was proposed as a non-destructive protocol for ZooMS, but in practice destructive samples are still taken for this protocol (see [4]). Submerging a sample in ammonium bicarbonate does chemically alter the ample, which is why current practices continue to take a destructive sample.
Non-destructive sampling protocols
Although the ammonium bicarbonate protocol should not be considered a non-destructive method, it was followed by more ‘true’ non-destructive methods. The first of these was the eraser protocol, first tested on parchment,[5] but later also applied to bone.[6] The eraser protocol is performed by rubbing a PVC eraser on a piece of parchment or bone. The friction generates triboelectric forces, which causes small particles of the sample to cling to the eraser waste. From the eraser waste collagen can then be extracted and analysed. The eraser protocol was found to work relatively well for parchment, but it is less effective on bone. Additionally, it leaves microscopic traces on the bone surface, which appear very similar to use wear traces and could be an issue for use wear analysis.[6]
A second non-destructive protocol is the plastic bag protocol, first published in 2019.[7] It is based on the idea that the normal friction between an object and the plastic bags, commonly used for storing archaeological objects, might be sufficient to extract enough material for ZooMS analysis.
A third protocol uses the same triboelectric principle. However, instead of using an eraser, this microgrid protocol employs a fine polishing film to remove very small amounts of material from a sample.[8]
The last non-destructive protocol that has been published for ZooMS is the membrane box protocol.[9] The membrane box protocol is based on contact electrification, which is the generation of electrostatic forces due to small localised differences in charge between two objects. These electrostatic forces can be large enough for material transfer between two surfaces.[10]
Most of these protocols have only been published recently and their respective advantages and disadvantages have not yet been tested against each other. It is therefore not yet clear how reliable these methods are and what level of preservation of the samples is required for them to work.
Reference biomarkers
Apart from non-destructive sampling, a second area of method development has been the expansion of reference biomarkers. To identify a species using ZooMS, a set of diagnostic biomarkers is used. These biomarkers correspond to particular fragments of the species’ collagen protein. The set of known biomarkers at the time of ZooMS’ original publication was relatively limited, but recent publications have been expanding this list. A regularly updated list of published biomarkers is maintained by the University of York and can be found here.
Principle of the method
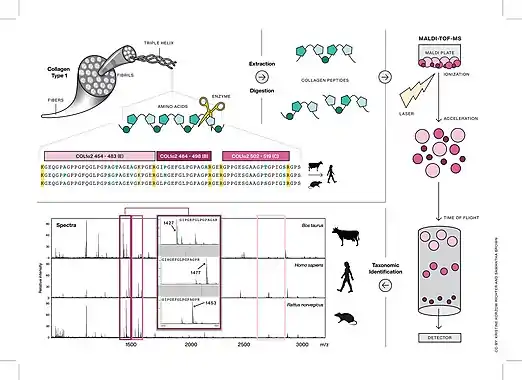
ZooMS identifies species based on differences in the amino acid composition of the collagen protein. The amino acid sequence of a species’ collagen protein is determined by its DNA and as a result like DNA, the amino acid sequence reflects a species’ evolutionary history. The greater the evolutionary distance between two species, the more different their collagen proteins will be. ZooMS typically can identify a sample up to genus level, though in some cases the identification can be more or less specific. A good understanding of the archaeological context of the sample can be used to further refine the resolution of the species identification.
Protocol example
A ZooMS protocol (Fig. 1) typically consists of an extraction, denaturation, digestion and filtration step, followed by mass spectrometric analysis. Various destructive and non-destructive extraction protocols have already been discussed in some detail above. The key is to extract the protein preserved in the sample and then bring it into solution, usually an ammonium bicarbonate buffer. Denaturation is done to unfold the proteins and make them more accessible for the enzymatic digestion. It is done by heating the solubilised sample at around 65 °C.[3] Then an enzyme, trypsin, is added to the solution. Trypsin cleaves the protein after every arginine or lysine amino acid in its sequence, resulting in peptide fragments of predictable masses. After digestion the sample is filtered with C18 filters to get rid of non-proteinaceous material and the sample is now ready for mass spectrometric analysis, which for ZooMS generally means MALDI-TOF MS.
References
- ↑ Buckley, Michael; Collins, Matthew; Thomas-Oates, Jane; Wilson, Julie C. (2009-12-15). "Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry: Species identification of bone collagen using MALDI-TOF-MS". Rapid Communications in Mass Spectrometry. 23 (23): 3843–3854. doi:10.1002/rcm.4316. PMID 19899187.
- ↑ Buckley, M., S. W. Kansa, S. Howard, S. Campbell, J. Thomas-Oates & M. J. Collins. 2010. Distinguishing between archaeological sheep and goat bones using a single collagen peptide. Journal of Archaeological Science 37: 13-20.
- 1 2 van Doorn, Nienke Laura; Hollund, Hege; Collins, Matthew J. (2011-09-01). "A novel and non-destructive approach for ZooMS analysis: ammonium bicarbonate buffer extraction". Archaeological and Anthropological Sciences. 3 (3): 281–289. doi:10.1007/s12520-011-0067-y. ISSN 1866-9565. S2CID 85056079.
- ↑ Naihui, Wang; Samantha, Brown; Peter, Ditchfield; Sandra, Hebestreit; Maxim, Kozilikin; Sindy, Luu; Oshan, Wedage; Stefano, Grimaldi; Michael, Chazan; Liora, Horwitz Kolska; Matthew, Spriggs; Glenn, Summerhayes; Michael, Shunkov; Kristine, Richter Korzow; Katerina, Douka (2021-02-20). "Testing the efficacy and comparability of ZooMS protocols on archaeological bone". Journal of Proteomics. 233: 104078. doi:10.1016/j.jprot.2020.104078. ISSN 1874-3919. PMID 33338688. S2CID 229325462.
- ↑ Fiddyment, Sarah; Holsinger, Bruce; Ruzzier, Chiara; Devine, Alexander; Binois, Annelise; Albarella, Umberto; Fischer, Roman; Nichols, Emma; Curtis, Antoinette; Cheese, Edward; Teasdale, Matthew D.; Checkley-Scott, Caroline; Milner, Stephen J.; Rudy, Kathryn M.; Johnson, Eric J. (2015-12-08). "Animal origin of 13th-century uterine vellum revealed using noninvasive peptide fingerprinting". Proceedings of the National Academy of Sciences. 112 (49): 15066–15071. doi:10.1073/pnas.1512264112. ISSN 0027-8424. PMC 4679014. PMID 26598667.
- 1 2 Sinet-Mathiot, Virginie; Martisius, Naomi L.; Schulz-Kornas, Ellen; van Casteren, Adam; Tsanova, Tsenka R.; Sirakov, Nikolay; Spasov, Rosen; Welker, Frido; Smith, Geoff M.; Hublin, Jean-Jacques (2021-12-08). "The effect of eraser sampling for proteomic analysis on Palaeolithic bone surface microtopography". Scientific Reports. 11 (1): 23611. doi:10.1038/s41598-021-02823-w. ISSN 2045-2322. PMC 8655045. PMID 34880290.
- ↑ McGrath, Krista; Rowsell, Keri; Gates St-Pierre, Christian; Tedder, Andrew; Foody, George; Roberts, Carolynne; Speller, Camilla; Collins, Matthew (2019-07-30). "Identifying Archaeological Bone via Non-Destructive ZooMS and the Materiality of Symbolic Expression: Examples from Iroquoian Bone Points". Scientific Reports. 9 (1): 11027. doi:10.1038/s41598-019-47299-x. ISSN 2045-2322. PMC 6667708. PMID 31363122.
- ↑ Kirby, Daniel P.; Manick, Annette; Newman, Richard (2020-10-01). "Minimally Invasive Sampling of Surface Coatings for Protein Identification by Peptide Mass Fingerprinting: A Case Study with Photographs". Journal of the American Institute for Conservation. 59 (3–4): 235–245. doi:10.1080/01971360.2019.1656446. ISSN 0197-1360. S2CID 210522155.
- ↑ Martisius, Naomi L.; Welker, Frido; Dogandžić, Tamara; Grote, Mark N.; Rendu, William; Sinet-Mathiot, Virginie; Wilcke, Arndt; McPherron, Shannon J. P.; Soressi, Marie; Steele, Teresa E. (2020-05-08). "Non-destructive ZooMS identification reveals strategic bone tool raw material selection by Neandertals". Scientific Reports. 10 (1): 7746. doi:10.1038/s41598-020-64358-w. ISSN 2045-2322. PMC 7210944. PMID 32385291.
- ↑ Galembeck, Fernando; Burgo, Thiago A. L.; Balestrin, Lia B. S.; Gouveia, Rubia F.; Silva, Cristiane A.; Galembeck, André (2014-11-24). "Friction, tribochemistry and triboelectricity: recent progress and perspectives". RSC Advances. 4 (109): 64280–64298. doi:10.1039/C4RA09604E. ISSN 2046-2069.
- ↑ Brown, Samantha; Douka, Katerina; Collins, Matthew J; Richter, Kristine Korzow (2021-03-20). "On the standardization of ZooMS nomenclature". Journal of Proteomics. 235: 104041. doi:10.1016/j.jprot.2020.104041. ISSN 1874-3919. PMID 33160104. S2CID 226279979.