乙腈性质表
乙腈的一些性质如下所述。
基本信息
- 中文名:乙
腈 - 化学式:CH3CN(或写作MeCN)
- CAS号:75-05-8
- 英文名:Acetonitrile
- 外观:无色液体
结构与性质
| 结构与性质 | |
|---|---|
| 折射率, nD | 1.344 at 20.0°C [1] |
| 阿贝数 | ? |
| 介电常数, εr | 36.64 ε0 at 20 °C [1] |
| 键偶极矩, | 3.84 D |
| 键强度 | ? |
| 键长 | ? |
| 键角 | ? |
| 磁化率 | ? |
| 黏度[2] | 0.441 mPa·s at 0°C 0.343 mPa·s at 25°C |
| 表面张力[2] | 29.29 dyn/cm |
热力学性质
| 相性质 | |
|---|---|
| 三相点[3] | 229.32 K (–43.83 °C), 167 Pa |
| 临界点 | 545 K (272 °C), 4.87 MPa |
| 标准熔化焓变, ΔfusH |
8.167 kJ/mol (crystal I → liq) |
| 标准熔化熵变, ΔfusS |
35.61 J/(mol·K) (crystal I → liq) |
| 标准汽化焓变, ΔvapH |
33.225 kJ/mol at 25°C 29.75 at 81.6°C (BP) |
| 标准汽化熵变, ΔvapS |
111.44 J/(mol·K) at 25°C |
| 固体性质 | |
| 标准摩尔生成焓, ΔfH |
? kJ/mol at 25°C |
| 标准摩尔熵, S |
? J/(mol K) |
| 热容量, cp | 92.36 J/(mol K)at 298.15 K |
| 标准相变焓变, ΔtrsH |
0.8979 kJ/mol at –56.2°C (crystal II → crystal I) |
| 标准相变熵变, ΔtrsS |
4.14 J/(mol·K) at –56.2° (crystal II → crystal I) |
| 液体性质 | |
| 标准摩尔生成焓, ΔfH |
–40.56 kJ/mol |
| 标准摩尔熵, S |
149.62 J/(mol K) |
| 燃烧热, ΔcH |
–1256.33 kJ/mol |
| 热容量, cp | 91.7 J/(mol K) at 25°C |
| 气体性质 | |
| 标准摩尔生成焓, ΔfH |
–74.04 kJ/mol |
| 标准摩尔熵, S |
? J/(mol K) |
| 燃烧热, cp | ? J/(mol K) |
液体蒸汽压
| 压力(mmHg) | 1 | 10 | 40 | 100 | 400 | 760 | |
| 温度(°C) | –47.0(s) | –16.3 | 7.7 | 27.0 | 62.5 | 81.8 | |
Table data obtained from CRC Handbook of Chemistry and Physics, 44th ed. The "(s)" notation indicates temperature of solid/vapor equilibrium. Otherwise the data is temperature of liquid/vapor equilibrium.
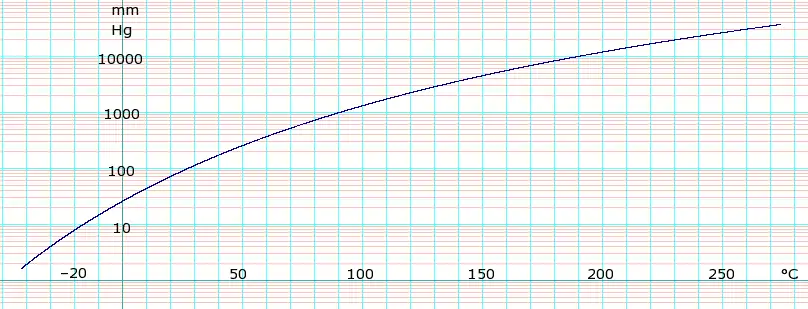
蒸馏数据
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
谱图数据
| UV-Vis | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λmax | ? nm | ||||||||||||||||||||||||||||||||||||
| 消光系数, ε | ? | ||||||||||||||||||||||||||||||||||||
| IR | |||||||||||||||||||||||||||||||||||||
| 主要吸收峰[5] |
| ||||||||||||||||||||||||||||||||||||
| NMR | |||||||||||||||||||||||||||||||||||||
| 1H NMR | |||||||||||||||||||||||||||||||||||||
| 13C NMR] | |||||||||||||||||||||||||||||||||||||
| 其它核磁共振数据 | |||||||||||||||||||||||||||||||||||||
| MS | |||||||||||||||||||||||||||||||||||||
| Masses of 主要碎片质量 |
|||||||||||||||||||||||||||||||||||||
化学反应方程式
| 反应物 | 反应方程式 | 反应条件 |
|---|---|---|
| 氧化亚铜、六氟磷酸 | Cu2O + 2 HPF6 + 8 CH3CN → 2 [Cu(CH3CN)4]PF6 + H2O | |
| 镍、氟硼酸亚硝酰 | Ni + 6 CH3CN + 2 NOBF4 → [Ni(CH3CN)6](BF4)2 + 2 NO | |
参考文献
- . [22 May 2014]. (原始内容存档于2017-10-19).
- (Queriable database). Chemical Engineering Research Information Center. [15 May 2007]. (原始内容存档于2007-06-03).
- Vapor Pressures of Acetonitrile Determined by Comparative Ebulliometry, Michael B. Ewing* and Jesus C. Sanchez Ochoa, Journal of Chemical & Engineering Data 2004 49 (3), 486-491
- . Chemical Engineering Research Information Center. [15 May 2007]. (原始内容 (Queriable database)存档于2007-08-29).
- . Advanced Industrial Science and Technology. [7 June 2007]. (原始内容 (Queriable database)存档于2006-05-05).
- . [15 May 2007]. (原始内容存档于2007-05-23).
不指明时,均指标准状态。其余信息参见Wikipedia:化学信息框。
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.