乙酸辛酯
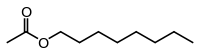
乙酸辛酯(英語:)是一种乙酸酯,化学式CH3COO(CH2)7CH3,可由1-辛醇和乙酸通过Fischer酯化反应合成[6][8][7]。在橙、葡萄柚等柑橘属中含有乙酸辛酯[10]。
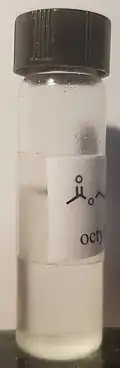
乙酸辛酯常温下为无色透明液体
| 乙酸辛酯 | |
|---|---|
 | |
 | |
 | |
| IUPAC名 Octyl ethanoate | |
| 别名 | Octyl acetate n-Octyl acetate |
| 识别 | |
| CAS号 | 112-14-1 |
| PubChem | 8164 |
| ChemSpider | 7872 |
| SMILES |
|
| InChI |
|
| InChIKey | YLYBTZIQSIBWLI-UHFFFAOYAX |
| ChEBI | 87495 |
| RTECS | AJ1400000 |
| 性质 | |
| 化学式 | C10H20O2 |
| 摩尔质量 | 172.26 g·mol−1 |
| 外观 | Colorless liquid |
| 氣味 | Fruity, slightly waxy floral odor |
| 密度 | 0.863–0.87 g/cm3[1][2] |
| 熔点 | -38.5--38 °C(273 K) |
| 沸点 | 203-211.3 °C(265 K) |
| 溶解性(水) | 0.021 g/100 g (0 °C) 0.018 g/100 g (29.7 °C) 0.018 g/100 g (40 °C) 0.012 g/100 g (92.1 °C)[3] |
| 溶解性 | Soluble in EtOH, ether |
| 蒸氣壓 | 0.01 kPa (−3 °C) 0.0072–0.0073 (14.75 °C) 0.02–0.1 kPa (27 °C)[4] 1 kPa (66.3 °C) 10 kPa (120 °C)[5] |
| 折光度n D |
1.415–1.422 (20 °C)[4] |
| 热力学 | |
| 热容 | 331–343.74 J/mol·K[6] |
| 危险性 | |
| NFPA 704 |
 2
1
0
|
| 爆炸極限 | 0.76–8.14%[7][8] |
| 致死量或浓度: | |
LD50(中位剂量) |
3000 mg/kg (oral, rat)[9] 5000 mg/kg (dermal, rabbit)[9] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- Record in the GESTIS Substance Database from the IFA
- Yaws, Carl L. . New York: William Andrew, Inc. 2008 [2020-04-23]. ISBN 978-0-8155-1596-8. LCCN 2008020146. (原始内容存档于2009-03-02).
- Stephenson, Richard M. . Journal of Chemical & Engineering Data. 1992, 37 (1): 80–95. doi:10.1021/je00005a024.
- . http://chemdats.blogspot.com. 2014-11-04 [2014-11-15]. (原始内容存档于2014-12-25). 外部链接存在于
|website=(帮助) - Lide, David R. (编). 90th. Boca Raton, Florida: CRC Press. 2009. ISBN 978-1-4200-9084-0 (英语).
- Acetic acid, octyl ester in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-11-22)
- . http://www.fishersci.ca. Fisher Scientific. [2014-09-15]. (原始内容存档于2014-11-29). 外部链接存在于
|website=(帮助) - 来源:Sigma-Aldrich Co., Octyl acetate (2014-11-15查阅).
- . Food and Cosmetics Toxicology. 1974, 12 (7–8): 815–816. doi:10.1016/0015-6264(74)90132-1.
- Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst. . . 2003. ISBN 978-3-527-30673-2. doi:10.1002/14356007.a11_141.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.