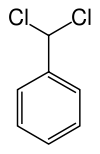
二氯化苄
二氯化苄是一种具有分子式:C6H5CHCl2的有机化合物。[2]
| 二氯化苄 | |||
|---|---|---|---|
| |||
| IUPAC名 Dichloromethylbenzene | |||
| 别名 | α,α-二氯甲苯[1] | ||
| 识别 | |||
| CAS号 | 98-87-3 | ||
| PubChem | 24855098 | ||
| ChemSpider | 13882337 | ||
| RTECS | CZ5075000 | ||
| KEGG | C19165 | ||
| 性质 | |||
| 化学式 | C7H6Cl2 | ||
| 161.03 g·mol⁻¹ | |||
| 外观 | Colorless liquid | ||
| 密度 | 1.254 g/cm3,液体 | ||
| 熔点 | −17 to −15 °C | ||
| 沸点 | 205 °C (82 °C @10 mm Hg) | ||
| 溶解性(水) | 微溶 | ||
| 蒸氣壓 | 0.6 kPa (45 °C) | ||
| 危险性 | |||
| 警示术语 | R:22-23-37/38-40-41 | ||
| 安全术语 | S:36/37-38-45 | ||
| 欧盟分类 | 毒性(T),致癌物質,对环境有害 (N) | ||
| 闪点 | 93 °C | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
二氯化苄可通过苯和氯气发生自由基反应制备,其过程还可以产生苄基氯和三氯甲苯:
- C6H5CH3 + Cl2 → C6H5CH2Cl + HCl
- C6H5CH2Cl + Cl2 → C6H5CHCl2 + HCl
- C6H5CHCl2 + Cl2 → C6H5CCl3 + HCl
- C6H5CHCl2 + H2O → C6H5CHO + 2 HCl
参考文献
- . terms.naer.edu.tw. 國家教育研究院. [2023-07-03]. (原始内容存档于2023-07-03).
- . International Programme on Chemical Safety. [2007-10-30]. (原始内容存档于2019-09-10). (页面存档备份,存于)
- Manfred Rossberg, Wilhelm Lendle, Gerhard Pfleiderer, Adolf Tögel, Eberhard-Ludwig Dreher, Ernst Langer, Heinz Rassaerts, Peter Kleinschmidt, Heinz Strack, Richard Cook, Uwe Beck, Karl-August Lipper, Theodore R. Torkelson, Eckhard Löser, Klaus K. Beutel, “Chlorinated Hydrocarbons” in Ullmann’s Encyclopedia of Chemical Technology, 2007 John Wiley & Sons: New York.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.