二氯硅烷
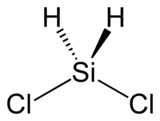
二氯硅烷,缩写DCS,是一种无机化合物,化学式为H2SiCl2。
| 二氯硅烷 | |||
|---|---|---|---|
 | |||
| |||
| IUPAC名 Dichlorosilane[1] | |||
| 别名 | 二氯甲硅烷 | ||
| 识别 | |||
| 缩写 | DCS | ||
| CAS号 | 4109-96-0 | ||
| PubChem | 61330 | ||
| ChemSpider | 55266 | ||
| SMILES |
| ||
| UN编号 | 2189 | ||
| EINECS | 223-888-3 | ||
| RTECS | VV3050000 | ||
| MeSH | dichlorosilane | ||
| 性质 | |||
| 化学式 | SiH 2Cl 2 | ||
| 101.007 g mol−1 g·mol⁻¹ | |||
| 外观 | 无色气体 | ||
| 密度 | 1.22 g cm−3 | ||
| 熔点 | −122 °C(151 K) | ||
| 沸点 | 8 °C(281.4 K) | ||
| 溶解性(水) | (反应) | ||
| 蒸氣壓 | 167.2 kPa (at 20 °C) | ||
| 热力学 | |||
| ΔfHm⦵298K | −320.49 kJ mol−1 | ||
| S⦵298K | 286.72 J K−1 mol−1 | ||
| 危险性 | |||
| 警示术语 | R:R12, R14, R26, R35 | ||
| 安全术语 | S:S26, S36/37/39, S45, S53, S60 | ||
| 欧盟分类 | |||
GHS危险性符号   | |||
| GHS提示词 | DANGER | ||
| H-术语 | H220, H250, H280, H314, H330 | ||
| P-术语 | P210, P261, P305+351+338, P310, P410+403 | ||
| NFPA 704 |
 4
4
2
| ||
| 爆炸極限 | 4.1–99% | ||
| 相关物质 | |||
| 相关化合物 | 氯硅烷 三氯硅烷 四氯化硅 | ||
| 相关化学品 | 二氯甲烷 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
参考资料
- . PubChem Compound. USA: National Center for Biotechnology Information. Identifiers and Related Records. 27 March 2005 [30 November 2011].
- 郝润蓉,方锡义,钮少冲. 《无机化学丛书》第三卷 碳 硅 锗 分族. 北京:科学出版社, 2011. P142. 2.3 卤代硅烷(SiHnX4-n). ISBN 978-7-03-030549-7
- Vorotyntsev, V., Mochalov, G., Kolotilova, M., Kinetics of Dichlorosilane Separation from a Mixture of Chlorosilanes by Distillation Using a Regular Packing, Theoretical Foundations of Chemical Engineering, 38(4), 355-359
- Vorotyntsev, V., Mochalov, G., Kolotilova, Volkova, E., Gas-Chromatographic and Mass-Spectrometric Determination of Impurity Hydrocarbons in Organochlorine Compounds and Dichlorosilane, Journal of Analytical Chemistry, 61(9), 883-888.
- Praxair Material Safety Data Sheet (2007)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.