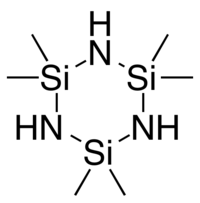
六甲基环三硅氮烷
六甲基环三硅氮烷,简称HMCTS[3]或HMCTSZN,[4]是一种有机化合物,化学式 C
6H
21N
3Si
3 或[–Si(CH
3)
2–NH–]
3。这个分子里面有由三个硅原子和三个氮原子交替排列而成的六元环,每个硅原子都连接两个甲基,氮原子则连接氢原子。它也可以看作是假想化合物环三硅氮烷 [–SiH
2–NH–]
3的衍生物,或是假想化合物二甲基硅氮烷 (CH
3)
2SiNH的三聚体。[2][5]
| 六甲基环三硅氮烷 | |
|---|---|
 | |
| IUPAC名 2,2,4,4,6,6-hexamethyl-1,3,5,2,4,6-triazatrisilinane | |
| 英文名 | |
| 别名 | 二甲基硅氮烷三聚体 三聚二甲基硅氮烷 HMCTS HCMTSZ |
| 识别 | |
| CAS号 | 1009-93-4 |
| PubChem | 66094 |
| ChemSpider | 59483 |
| SMILES |
|
| 性质 | |
| 化学式 | C6H21N3Si3 |
| 摩尔质量 | 219.51 g·mol−1 |
| 密度 | d20 0.9196 g/mL [1] |
| 熔点 | −10 °C [1][2] |
| 沸点 | 188 °C [1][2] |
| 折光度n D |
n20/D 1.445 [1] |
| 危险性 | |
GHS危险性符号   | |
| GHS提示词 | Danger |
| H-术语 | H226, H302, H314, H315, H318, H319, H335 |
| P-术语 | P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+312, P301+330+331 |
| 致死量或浓度: | |
LD50(中位剂量) |
500 mg/kg(大鼠)[2] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
六甲基环三硅氮烷在室温下是无色清澈液体。[2]
由于其在半导体工业中的应用,六甲基环三硅氮烷作为氮化硅[6][7][4]和碳氮化硅[8]沉积的前体已被广泛研究,并作为光刻胶中的添加剂。也有人建议将其作为二氧化硅的添加剂,用于色谱法。[9]
结构
六甲基环三硅氮烷的环几乎是一个平面。这个分子内的键长为 Si-N = 1.728 Å、Si-C = 1.871 Å、C-H = 1.124 Å。它的键角则为 N-Si-N ≈ 108°、Si-N-Si ≈ 127°、 C-Si-C ≈ 109°、H-C-H ≈ 112°。[10][11]
制备
六甲基环三硅氮烷是在1948年由 Brewer 和 Haber 通过二甲基二氯硅烷 Si(CH
3)
2Cl
2和液氨 NH
3反应,然后用苯萃取沉淀首次合成的。反应会产生多种产物,主要是三聚体和四聚体八甲基环四硅氮烷。三聚体可以通过分馏与其他产物分离。[1]
参见
参考资料
- Stuart D. Brewer and Charles P. Haber (1948): "Alkylsilazanes and Some Related Compounds". Journal of the American Chemical Society, volume 70, issue 11, pages 3888-3891. doi:10.1021/ja01191a106
- NCBI, U.S. National Institutes of Health (2020): "2,2,4,4,6,6-Hexamethylcyclotrisilazane". Compound data sheet, PubChem online database. Accessed on 2020-01-04.
- FAR Chemical (2020): "Product 654201: Hexamethylcyclotrisilazane (页面存档备份,存于)". Catalog page. Accessed on 2020-01-04.
- T. A. Brooks and D. W. Hess (1988): "Deposition chemistry and structure of plasma‐deposited silicon nitride films from 1,1,3,3,5,5‐hexamethylcyclotrisilazane". Journal of Applied Physics, volume 64, page 841. doi:10.1063/1.341935
- Beilstein 4 III 1887.
- Dennis W. Hess and Todd A. Brooks (1987): "Plasma enhanced chemical vapor deposition of thin films of silicon nitride from cyclic organosilicon nitrogen (页面存档备份,存于)". United States Patent 4863755A.
- T. A. Brooks and D. W. Hess (1987): "Plasma-enhanced chemical vapor deposition of silicon nitride from 1,1,3,3,5,5-hexamethylcyclotrisilazane and ammonia". Thin Solid Films, volume 153, issues 1–3, pages 521-529. doi:10.1016/0040-6090(87)90211-2
- N. I. Fainer, A. N. Golubenko, Yu. M. Rumyantsev, and E. A. Maximovskii (2009): "Use of hexamethylcyclotrisilazane for preparation of transparent films of complex compositions". Glass Physics and Chemistry, volume 35, issue 3, pages 274–283. doi:10.1134/S1087659609030067
- J. Nawrocki (1985): "Modification of silica with mixtures of hexamethylcyclotrisilazane and hexamethyldisilazane". Chromatographia´ volume 20, pages 308–312. doi:10.1007/BF02310388
- Béla Rozsondai, István Hargittai, Aleksei V. Golubinskii, Lev V.Vilkov, and Vladimir S.Mastryukov (1975): "Electron diffraction study on the molecular structure of hexamethylcyclotrisilazane, [(CH
3)
2SiNH]
3". Journal of Molecular Structure, volume 28, issue 2, pages 339-348. doi:10.1016/0022-2860(75)80104-9 - D. M. Adams and W. S. Fernando (1973): "The vibrational spectra of hexamethylcyclotrisiloxane and hexamethylcyclotrisilazane". Journal of the Chemical Society, Dalton Transactions, issue 4, pages 410-413.doi:10.1039/DT9730000410
- Hiromi Ohsaki, Yoshihumi Takeda, Toshinobu Ishihara, and Akira Hayashida (1991) "Method for preparing hexamethyl cyclotrisilazane (页面存档备份,存于)". United States Patent 5075474A
- Barry C. Arkles and Burrell N. Hamon (1985): "Method of preparing hexamethylcyclotrisilazane (页面存档备份,存于)" United States Patent 4577039A.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.