右啡烷
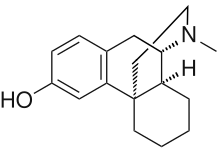
右啡烷(Dextrorphan,DXO)是一種吗啡類的精神药物,具有鎮咳與止咳,或作為解离性致幻剂的作用,也是外消旋嗎汎的右旋对映体,另一個左旋对映体則是左旋嗎汎。右啡烷是右美沙芬由CYP2D6酶经O-脱甲基(O-demethylated)而生成的,作為一种NMDA拮抗剂,右啡烷可产生右美沙芬所帶來的精神效應。[2]
 | |
 | |
| 臨床資料 | |
|---|---|
| 其他名稱 | DXO, Dextrorphanol |
| ATC碼 |
|
| 法律規範狀態 | |
| 法律規範 |
|
| 识别 | |
| |
| CAS号 | 125-73-5 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.323 |
| 化学 | |
| 化学式 | C17H23NO |
| 摩尔质量 | 257.38 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
藥理學
藥效學
| 位置 | Ki (nM) | 對象 | 引用 |
|---|---|---|---|
| NMDAR (MK-801) | 486–906 | 鼠 | [4] |
| σ1 | 118–481 | 鼠 | [4] |
| σ2 | 11,325–15,582 | 鼠 | [4] |
| MOR | 420 >1,000 | 鼠 人 | [4][7] |
| DOR | 34,700 | 鼠 | [4] |
| KOR | 5,950 | 鼠 | [4] |
| SERT | 401–484 | 鼠 | [4] |
| NET | ≥340 | 鼠 | [4] |
| DAT | >1,000 | 鼠 | [4] |
| 5-HT1A | >1,000 | 鼠 | [4] |
| 5-HT1B/1D | 54% at 1 μM | 鼠 | [4] |
| 5-HT2A | >1,000 | 鼠 | [4] |
| α1 | >1,000 | 鼠 | [4] |
| α2 | >1,000 | 鼠 | [4] |
| β | 35% at 1 μM | 鼠 | [4] |
| D2 | >1,000 | 鼠 | [4] |
| H1 | 95% at 1 μM | 鼠 | [4] |
| mAChRs | 100% at 1 μM | 鼠 | [4] |
| nAChRs | 1,300–29,600 (IC50) | 鼠 | [4] |
| VDSCs | ND | ND | ND |
| 除非另加说明,否则以上所示数值均为Ki(nM)。数值越小,說明药物与该位点的结合力越强。 | |||
右啡烷的药理作用類似於右美沙芬。不过,右啡烷作为NMDA受体拮抗剂的效力要强得多,作为SRI的活性也低得多。不過右啡烷仍保留了右美沙芬作为NRIs時的活性。[8]並且其对阿片受体的亲和力也較右美沙芬强,且高剂量下更強。
參考文獻
- Bensinger, Peter. (PDF). NARA. October 1, 1976 [June 26, 2023]. (原始内容存档 (PDF)于2023-06-27).
- Zawertailo LA, Kaplan HL, Busto UE, Tyndale RF, Sellers EM. . Journal of Clinical Psychopharmacology. August 1998, 18 (4): 332–337. PMID 9690700. doi:10.1097/00004714-199808000-00014.
- Roth BL, Driscol J. . Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. [14 August 2017]. (原始内容存档于2023-01-01).
- Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR. . Pharmacol. Ther. 2016, 159: 1–22. PMID 26826604. doi:10.1016/j.pharmthera.2016.01.016.
- Werling LL, Keller A, Frank JG, Nuwayhid SJ. . Exp. Neurol. 2007, 207 (2): 248–57. PMID 17689532. S2CID 38476281. doi:10.1016/j.expneurol.2007.06.013.
- Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR. . Pharmacol. Ther. 2016, 164: 170–82. PMID 27139517. doi:10.1016/j.pharmthera.2016.04.010
 .
. - Raynor K, Kong H, Mestek A, Bye LS, Tian M, Liu J, Yu L, Reisine T. . J. Pharmacol. Exp. Ther. 1995, 272 (1): 423–8. PMID 7815359.
- Pechnick RN, Poland RE. . The Journal of Pharmacology and Experimental Therapeutics. May 2004, 309 (2): 515–522. PMID 14742749. S2CID 274504. doi:10.1124/jpet.103.060038.
- Yu A, Haining RL. . Drug Metabolism and Disposition. November 2001, 29 (11): 1514–20 [2023-10-01]. PMID 11602530. (原始内容存档于2020-03-12).
- . [2023-10-01]. (原始内容存档于2021-07-05).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.