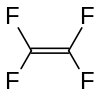
四氟乙烯
四氟乙烯,结构式F2C=CF2,是最简单的全氟烯烃。这种气体主要用于生产氟碳涂料。
| 四氟乙烯 | |||
|---|---|---|---|
| |||
| IUPAC名 Tetrafluoroethene | |||
| 别名 | 1,1,2,2-四氟乙烯 全氟乙烯 R1114 TFE | ||
| 识别 | |||
| CAS号 | 116-14-3 | ||
| PubChem | 8301 | ||
| ChemSpider | 8000 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | BFKJFAAPBSQJPD-UHFFFAOYAC | ||
| ChEBI | 38866 | ||
| KEGG | C19299 | ||
| 性质 | |||
| 化学式 | C2F4 | ||
| 摩尔质量 | 100.02 g·mol−1 | ||
| 外观 | 无色气体[1] | ||
| 密度 | 1.519 g/cm3(液态,-76 °C) 4.51 g/l (气态,0 °C)[1] | ||
| 熔点 | -142.5 °C[1] | ||
| 沸点 | -75.6 °C[1] | ||
| 溶解性(水) | 179 mg/l (20 °C)[1] | ||
| 蒸氣壓 | 2.95 MPa (20 °C)[1] | ||
| 热力学 | |||
| ΔfHm⦵298K | −658.9 kJ·mol−1[2] | ||
| 危险性 | |||
| 警示术语 | R:R12 | ||
| 安全术语 | S:S9, S16, S33 | ||
| 欧盟分类 | 极易燃 (F+) | ||
| NFPA 704 |
 4
3
3
OX
| ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
制备
参考资料
- Record of Tetrafluorethen in the GESTIS Substance Database from the IFA
- David R. Lide (Hrsg.): CRC Handbook of Chemistry and Physics. 90. Auflage. (Internet-Version: 2010), CRC Press/Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, S. 5-21.
- Gozzo, F.; Camaggi, G. . Tetrahedron. January 1966, 22 (6): 1765–1770. doi:10.1016/S0040-4020(01)82248-1.
- Sung, Dae Jin; Moon, Dong Ju; Lee, Yong Jun; Hong, Suk-In. . International Journal of Chemical Reactor Engineering (Walter de Gruyter GmbH). 2004-01-28, 2 (1). ISSN 1542-6580. doi:10.2202/1542-6580.1065.
- Ruff, Otto; Bretschneider, Otto. . Zeitschrift für anorganische und allgemeine Chemie (Wiley). 1933-01-13, 210 (2): 173–183. ISSN 0863-1786. doi:10.1002/zaac.19332100212.
- Hercules, Daniel A.; Parrish, Cameron A.; Sayler, Todd S.; Tice, Kevin T.; Williams, Shane M.; Lowery, Lauren E.; Brady, Michael E.; Coward, Robert B.; Murphy, Justin A.; Hey, Trevyn A.; Scavuzzo, Anthony R.; Rummler, Lucy M.; Burns, Emory G.; Matsnev, Andrej V.; Fernandez, Richard E.; McMillen, Colin D.; Thrasher, Joseph S. . Journal of Fluorine Chemistry. 2017, 196: 107–116. doi:10.1016/j.jfluchem.2016.10.004.
- R. J. Hunadi; K. Baum. . Synthesis. 1982, 39 (6): 454. doi:10.1055/s-1982-29830.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.