氯乙炔
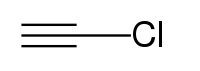
氯乙炔是一种有机化合物,化学式为HC≡CCl。它可在氯乙酰氯和三氯化铝的共热中产生。[3]它也可以由氯乙炔汞(Hg(C≡CCl)2)和氰化钾的氢氧化钾溶液反应得到。[4]氯乙炔接触空气自燃[4]或者爆炸[5]。它和银氨溶液反应,生成具有高爆炸性的氯乙炔银。[6]
| 氯乙炔 | |
|---|---|
 | |
| 英文名 | |
| 别名 | 一氯乙炔 氯代乙炔 |
| 识别 | |
| CAS号 | 593-63-5 |
| SMILES |
|
| 性质 | |
| 化学式 | C2HCl |
| 60.48 g·mol⁻¹ | |
| 密度 | 1.066±0.06 g·cm−3(20 °C)[1] |
| 熔点 | −126 °C(147 K)[2] |
| 沸点 | −30 °C(243 K)[2] |
| 溶解性(水) | 0.34 g(25 °)[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2019 ACD/Labs). Retrieved from SciFinder. [2019-12-5]
- "PhysProp" data were obtained from Syracuse Research Corporation of Syracuse, New York (US). Retrieved from SciFinder. [2019-12-6]
- Pomerantseva, E. G.; Kulikova, A. E.; Zil'berman, E. N. Decarbonylation of chloroacetic acid chlorides in the presence of aluminum chloride(俄文). Zhurnal Organicheskoi Khimii, 1969. 5 (1): 187. ISSN: 0514-7492.
- William E. Truce, Max M. Boudakian, Richard F. Heine, Robert J. McManimie. . Journal of the American Chemical Society. 1956-06, 78 (12): 2743–2748 [2019-12-06]. ISSN 0002-7863. doi:10.1021/ja01593a026. (原始内容存档于2020-02-02) (英语).
- Mei-Lee Hwang, Wei-Liang Hsu. . Journal of Molecular Structure. 1998-08, 449 (1): 29–37 [2019-12-06]. doi:10.1016/S0022-2860(98)00361-5. (原始内容存档于2020-02-02) (英语).
- K. A. Hofmann, H. Kirmreuther. . Berichte der deutschen chemischen Gesellschaft. 1909-11, 42 (4): 4232–4238 [2019-12-06]. doi:10.1002/cber.19090420404 (德语).
拓展阅读
- Cheng Zhu, Markku Räsänen, Leonid Khriachtchev. . The Journal of Chemical Physics. 2015-12-28, 143 (24): 244319 [2019-12-06]. ISSN 0021-9606. doi:10.1063/1.4938426. (原始内容存档于2020-02-02) (英语).
- Е. P. Levanova, V. S. Nikonova, V. А. Grabel’nykh, N. V. Russavskaya, A. I. Albanov, I. B. Rozentsveig, N. А. Korchevin. . Russian Journal of General Chemistry. 2018-03, 88 (3): 383–388 [2019-12-06]. ISSN 1070-3632. doi:10.1134/S1070363218030015 (英语).
- William A. Arnold, A. Lynn Roberts. . Environmental Science & Technology. 2000-05, 34 (9): 1794–1805 [2019-12-06]. ISSN 0013-936X. doi:10.1021/es990884q. (原始内容存档于2020-02-02) (英语).
- William A. Arnold, A. Lynn Roberts. . Environmental Science & Technology. 1998-10, 32 (19): 3017–3025 [2019-12-06]. ISSN 0013-936X. doi:10.1021/es980252o. (原始内容存档于2020-02-02) (英语).
- Jian-Wei Zou, Yong-Jun Jiang, Ming Guo, Gui-Xiang Hu, Bing Zhang, Hai-Chun Liu, Qing-Sen Yu. . Chemistry - A European Journal. 2005-01-07, 11 (2): 740–751 [2019-12-06]. ISSN 0947-6539. doi:10.1002/chem.200400504 (英语).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.