烟碱烯
烟碱烯是一种有机化合物,化学式为C10H10N2。它可由烟碱的催化脱氢反应制得;[3]1-甲基吡咯和3-溴吡啶在二异丙基乙胺存在下、9,10-二氰基蒽的催化下于乙腈中反应,也能得到产物。[4]
| 烟碱烯 | |
|---|---|
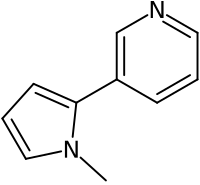 | |
| IUPAC名 3-(1-methylpyrrol-2-yl)pyridine | |
| 别名 | 二烯烟碱 尼古提林 β-烟碱烯 1-甲基-2-(3-吡啶基)吡咯 3-(1-甲基-2-吡咯基)吡啶 |
| 识别 | |
| CAS号 | 487-19-4 |
| PubChem | 10249 |
| ChemSpider | 2282468 |
| SMILES |
|
| ChEBI | 7564 |
| KEGG | C10161 |
| 性质 | |
| 化学式 | C10H10N2 |
| 摩尔质量 | 158.2 g·mol−1 |
| 熔点 | 168—169 °C(441—442 K)[1] |
| 沸点 | 281 °C(554 K)[2] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参见
- 麦斯明
参考文献
- Spath, Ernst; Tobacco alkaloids. XIII. New tobacco bases. Berichte der Deutschen Chemischen Gesellschaft [Abteilung] B: Abhandlungen, 1937. 70B: 2450-2454. ISSN: 0365-9488.
- "PhysProp" data were obtained from Syracuse Research Corporation of Syracuse, New York (US). Retrieved from SciFinder. [2021-08-14].
- Makoto Shibagaki, Kyoko Takahashi, Hideyuki Kuno, Hajime Matsushita. . Agricultural and Biological Chemistry. 1988-10, 52 (10): 2651–2652 [2021-08-14]. ISSN 0002-1369. doi:10.1080/00021369.1988.10869102 (英语).
- Michael Neumeier, Diego Sampedro, Michal Májek, Víctor A. de la Peña O'Shea, Axel Jacobi von Wangelin, Raúl Pérez-Ruiz. . Chemistry - A European Journal. 2018-01-02, 24 (1): 105–108 [2021-08-14]. doi:10.1002/chem.201705326 (英语).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.