甲烯𬭩离子
在有机化学中,甲烯鎓離子(又稱质子化亚甲基)是一种阳离子,化学式为CH+
3。它可以视作一个亚甲基自由基(:CH
2)加上一个质子(H+
),或一个甲基自由基(•CH
3)去除一个电子。它是碳正离子和烯𬭩离子,是最简单的烯𬭩离子。[2]
| 甲烯𬭩离子 | |
|---|---|
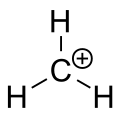 | |
| IUPAC名 Methylium[1] | |
| 别名 | Methyl cation; Carbanylium |
| 识别 | |
| CAS号 | 14531-53-4 |
| PubChem | 644094 |
| SMILES |
|
| InChI |
|
| InChIKey | UHDUIDUEUEQND-UHFFFAOYSA-N |
| ChEBI | 29437 |
| 性质 | |
| 化学式 | CH3 |
| 摩尔质量 | 15.03 g·mol−1 |
| 相关物质 | |
| 相关等离子化合物 | 甲硼烷 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
制备和反应
对于低压的质谱研究,甲烯鎓離子可以通过甲基自由基的紫外线光电离,[2]或将中性甲烷与单原子阳离子(如C+
和Kr+
)碰撞制备。[3]在这种条件下,它会与乙腈CH
3CN反应,形成离子(CH
3)
2CN+
。[4]
它很少在凝聚相下以中间体的形式出现。它被提议为一种反应性中间体,在甲烷与FSO3H-SbF5进行质子化或氢化物提取时形成。甲鎓离子非常活泼,对烷烃也是如此。[6]
参考文献
- International Union of Pure and Applied Chemistry. . The Royal Society of Chemistry. 2014: 1089. ISBN 978-0-85404-182-4. doi:10.1039/9781849733069.
- L. Golob, N. Jonathan, A. Morris, M. Okuda, K.J. Ross (1972), "The first ionization potential of the methyl radical as determined by photoelectron spectroscopy". Journal of Electron Spectroscopy and Related Phenomena, volume 1, issue 5, pp. 506–508 doi:10.1016/0368-2048(72)80022-7
- R.B. Sharma, N.M. Semo, W.S. Koski (1987), "Dynamics of the reactions of methylium, methylene radical cation, and methyliumylidene with acetylene". Journal of Physical Chemistry, volume 91, issue 15, pp. 4127–4131 doi:10.1021/j100299a037
- Murray J. McEwan, Arthur B. Denison, Wesley T. Huntress Jr., Vincent G. Anicich, J. Snodgrass, M.T. Bowers (1989), "Association reactions at low pressure. 2. The methylium/methyl cyanide system". Journal of Physical Chemistry, volume 93, issue 10, pp. 4064–4068. doi:10.1021/j100347a039
- E.M. Bahati, M. Fogle, C.R. Vane, M.E. Bannister, R.D. Thomas and V. Zhaunerchyk (2009), "Electron-impact dissociation of CD+
3 and CH+
3 ions producing CD+
2, CH+
and C+
fragment ions". Physical Review, volume 79, article 052703 doi:10.1103/PhysRevA.79.052703 - Hogeveen, H.; Lukas, J.; Roobeek, C. F. . Journal of the Chemical Society D: Chemical Communications. 1969, (16): 920. ISSN 0577-6171. doi:10.1039/c29690000920 (英语).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.