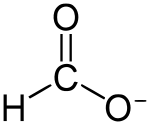
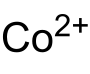甲酸钴
甲酸钴是钴(II)的甲酸盐,化学式为Co(HCOO)2(或写作Co(HCO2)2)。可用于制备其它钴催化剂。
| 甲酸钴 | ||
|---|---|---|
| ||
| IUPAC名 Cobalt(II) formate | ||
| 识别 | ||
| CAS号 | 544-18-3 | |
| PubChem | 10998 | |
| ChemSpider | 10532 | |
| SMILES |
| |
| InChI |
| |
| InChIKey | PFQLIVQUKOIJJD-NUQVWONBAE | |
| EINECS | 208-862-4 | |
| 性质 | ||
| 化学式 | Co(HCOO)2 | |
| 148.971 g/mol(无水) 185.003 g/mol(二水) g·mol⁻¹ | ||
| 外观 | 红色晶体 | |
| 密度 | 2.13 g/cm3 (20 °C) | |
| 熔点 | 175℃(分解) | |
| 溶解性(水) | 可溶 | |
| 溶解性 | 难溶于乙醇 | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | ||
参考文献
- GR Rao,KC Patil,CNR Rao. Spectra and thermal decompositions of metal formates. Inorganica Chimica Acta, 1970, 4(00): 215–218
拓展阅读
- Xin Wang, Lude Lu, Peicheng Wu. Reactions of cobalt formate on supports[J]. Thermochimica Acta, 1990. 165(1): 139-145
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.