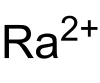硝酸镭
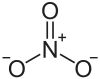
硝酸镭是一种无机化合物,化学式为Ra(NO3)2,有强放射性。它是白色固体,但旧样本呈黄灰色。它的溶解度比硝酸钡低。[1][4]
| 硝酸镭 | |||
|---|---|---|---|
 | |||
| |||
| 英文名 | |||
| 识别 | |||
| CAS号 | 10213-12-4 | ||
| PubChem | 56844152 | ||
| SMILES |
| ||
| 性质 | |||
| 化学式 | Ra(NO3)2 | ||
| 350.0343 g·mol⁻¹ | |||
| 外观 | 白色固体[1] | ||
| 密度 | 4.176 g/cm3(100 K[2] | ||
| 溶解性(水) | 12.1g(20℃)[3] | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
参考文献
- Otto Erbacher. [Solubility determinations of some radium salts]. Chemische Berichte. 1930, 63 (1): 141–156. doi:10.1002/cber.19300630120 (德语).
- Zhuanling Bai, Jacob Brannon, Cristian Celis-Barros, Nicholas Beck, Joseph M. Sperling, Brian M. Rotermund, Daniela Gomez Martinez, Hannah B. Wineinger, and Thomas E. Albrecht-Schönzart. Radium Revisited: Revitalization of the Coordination Chemistry of Nature’s Largest +2 Cation. Inorg. Chem. 2023, 62, 22, 8478–8481. doi:10.1021/acs.inorgchem.3c01170.
- Richard C. Ropp. Encyclopedia of the Alkaline Earth Compounds. Elsevier, 2013. pp 223. Radium Nitrate
- Kirby 1964,第4–8頁
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.