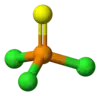
三氯硫磷
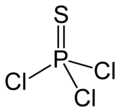
三氯硫磷,化学式PSCl3。
| 三氯硫磷[1] | |||
|---|---|---|---|
 | |||
| |||
| IUPAC名 Thiophosphoryl chloride | |||
| 识别 | |||
| CAS号 | 3982-91-0 | ||
| PubChem | 19883 | ||
| ChemSpider | 18729 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | WQYSXVGEZYESBR-UHFFFAOYAE | ||
| 性质 | |||
| 化学式 | Cl3PS | ||
| 169.40 g·mol⁻¹ | |||
| 外观 | 无色有挥发性液体,有刺激性气味 | ||
| 密度 | 1.668 g/cm³ | ||
| 熔点 | -40.8 °C (α型) -36.2 °C (β型) | ||
| 沸点 | 125 °C | ||
| 溶解性(水) | 遇水分解 | ||
| 溶解性(其他) | 易溶于多种有机溶剂,如苯、氯仿及二硫化碳[2] | ||
| 蒸氣壓 | 16 hPa (20 °C) | ||
| 危险性 | |||
| 警示术语 | R:R34, R37, R52, R53 | ||
| 安全术语 | S:S7/8, S26 | ||
| 欧盟分类 | 腐蚀性 (C) | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
用途
三氯硫磷主要用于生产有机磷农药如甲基对硫磷、二甲基硫代磷酰氯、二乙基硫代磷酰氯以及甲胺磷、倍硫磷、禾螟松等高效低毒有机磷农药的原料。[6] 也用于有机合成中向底物分子[3] 引入 P=S 键,制取含磷和含硫化合物。例如,三氯硫磷与三级酰胺反应,得到相应的硫代酰胺。[2]
参考资料
- Record of CAS RN 3982-91-0 in the GESTIS Substance Database from the IFA
- Spilling, C. D. "Thiophosphoryl Chloride" in Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, Weinheim, 2001. doi: 10.1002/047084289X.rt104. Article Online Posting Date: April 15, 2001.
- 中国化工产品大全 上卷,Bc238 三氯硫磷,页224
- Betterman, G.; Krause, W.; Riess, G.; Hofmann, T. "Phosphorus Compounds, Inorganic" Ullman's Encyclopedia of Industrial Chemistry. John Wiley & Sons: New York, 2005. doi: 10.1002/14356007.a19_527.
- Martin, D. R.; Duvall, W. M. “Phosphorus (V) Sulfochloride” Inorganic Syntheses, Volume IV. McGraw-Hill, 1953. doi: 10.1002/9780470132357.ch24.
- Fee, D. C.; Gard, D. R.; Yang, C. “Phosphorus Compounds” Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons: New York, 2005. doi: 10.1002/0471238961.16081519060505.a01.pub2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.