硫化铍
| 硫化铍 | |
|---|---|
 | |
| 英文名 | |
| 识别 | |
| CAS号 | 13598-22-6 |
| PubChem | 83605 |
| ChemSpider | 44415277 |
| SMILES |
|
| InChI |
|
| InChIKey | FQDSYGKTHDFFCM-UHFFFAOYSA-N |
| EINECS | 237-064-6 |
| 性质 | |
| 化学式 | BeS |
| 41.077 g·mol⁻¹ | |
| 外观 | 白色晶体 |
| 密度 | 2.36 g/cm3 |
| 熔点 | 1,800 °C(2,070 K)(分解) |
| 能隙 | 7.4 eV |
| 折光度n D |
1.741 |
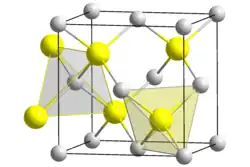
| 结构 | |
| 晶体结构 | cubic |
| 热力学 | |
| ΔfHm⦵298K | -235 kJ/mol |
| S⦵298K | 34 J/mol K |
| 热容 | 34 J/mol K |
| 危险性 | |
| PEL | TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- NIOSH Pocket Guide to Chemical Hazards #0054". National Institute for Occupational Safety and Health (NIOSH).
- Kenneth A. Walsh. . ASM International. 2009: 127 [2017-06-24]. ISBN 087170721-7. (原始内容存档于2016-12-23).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.