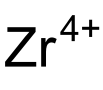硫酸锆
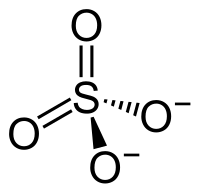
硫酸锆是一种无机化合物,化学式为Zr(SO4)2,它可以以无水物、四水合物、五水合物和七水合物的形式存在。它们是白色可溶于水的固体。
| 硫酸锆 | ||
|---|---|---|
| ||
| 英文名 | ||
| 别名 | 二硫酸锆 硫酸锆(IV) | |
| 识别 | ||
| CAS号 | 14644-61-2(无水) 7446-31-3(四水) | |
| PubChem | 26793(无水) 53249516(一水) | |
| SMILES |
| |
| RTECS | ZH9100000 | |
| 性质 | ||
| 化学式 | Zr(SO4)2(H2O)x ( x = 0, 4, 5, 7) | |
| 285.35 g/mol(无水) 355.40(四水) g·mol⁻¹ | ||
| 外观 | 白色固体 | |
| 密度 | 3.22 g/cm3(无水) 2.85 g/cm3(四水) | |
| 溶解性(水) | 52.5 g(四水) | |
| 折光度n D |
1.646 | |
| 结构 | ||
| 晶体结构 | 正交晶系 | |
| 危险性 | ||
| 致死量或浓度: | ||
LD50(中位剂量) |
3500 mg/kg(大鼠,口服)[1] | |
| 相关物质 | ||
| 其他阳离子 | 硫酸钛 | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | ||
制备与结构
- ZrO2 + 2 H2SO4 + x H2O → Zr(SO4)2·xH2O
参考文献
- "Zirconium compounds (as Zr)". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH)
- Bear, Isabel J.; Mumme, W. G. "Crystal chemistry of zirconium sulfate. III. Structure of the β-pentahydrate, Zr2(SO4)4(H2O)8.2H2O, and the interrelationship of the four higher hydrates" Acta Crystallogr. 1969. B25, 1572-1581. doi:10.1107/S0567740869004341
- Squattrito, Philip J.; Rudolf, Philip R.; Clearfield, Abraham "Crystal structure of a complex basic zirconium sulfate" Inorganic Chemistry 1987, vol. 26, 4240-4.doi:10.1021/ic00272a020
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.