臭氧化钾
臭氧化钾是一种无机化合物,化学式为KO3。它是橙红色的四方晶系晶体,空间群I4/mcm。[7]
| 臭氧化钾 | |
|---|---|
 | |
| 英文名 | |
| 识别 | |
| CAS号 | 12030-89-6 |
| SMILES |
|
| 性质 | |
| 化学式 | KO3 |
| 摩尔质量 | 87.1 g·mol−1 |
| 外观 | 橘红色晶体[1] |
| 密度 | 1.990 g/cm3[2] |
| 熔点 | 25 °C(298 K)(分解[3]) |
| 溶解性(水) | 反应 |
| 溶解性 | 可溶于液氨[4] |
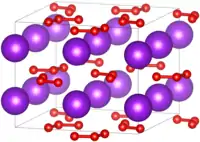
| 结构[5][6] | |
| 空间群 | I4/mcm |
| 晶格常数 | a = 8.597 Å, c = 7.080 Å |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
制备
臭氧化钾可由氢氧化钾和臭氧反应得到,[1]但产率很低,只有5-10%。[8]
- 6 KOH + 4 O3 → 4 KO3 + 2 KOH·H2O + O2
超氧化钾用臭氧-氧气的混合物(体积比2:98)进行臭氧化,也能得到臭氧化钾。[9]
- KO2 + O3 → KO3 + O2
性质
- 4 KO3 + 2 H2O → 4 KOH + 5 O2↑
臭氧化钾不稳定,在室温时即可缓慢分解:[1]
- 2 KO3 → 2 KO2 + O2↑
它易溶于液氨,并有部分缓慢反应:
- 2 KO3 + 2 NH3 → NH4O3 + KNH2
参考文献
- 无机化学丛书. 第一卷 稀有气体 氢 碱金属. 科学出版社. pp 421-422. 4. 臭氧化物
- Sokol, V. I.; Tokareva, S. A.; Sokovnin, E. I. . Bulletin of the Academy of Sciences, USSR Division of Chemical Science (Springer Science and Business Media LLC). 1963, 12 (12): 2051–2052. ISSN 0568-5230. doi:10.1007/bf00844013.
- Petrocelli, W.; Capotosto, A. . Inorganica Chimica Acta (Elsevier BV). 1971, 5: 453–456. ISSN 0020-1693. doi:10.1016/s0020-1693(00)95963-2.
- Makarov, S. Z.; Sokovnin, E. I. [Solubility of potassium ozonide in liquified ammonia]. Proceedings of the USSR Academy of Sciences. 1961, 137 (3): 612–613 (俄语). English translation (页面存档备份,存于)
- Azaroff, L. V.; Corvin, I. . Proceedings of the National Academy of Sciences. 1963-01-01, 49 (1): 1–5. Bibcode:1963PNAS...49....1A. ISSN 0027-8424. JSTOR 71636. PMC 300616
 . PMID 16591034. doi:10.1073/pnas.49.1.1
. PMID 16591034. doi:10.1073/pnas.49.1.1  .
. - Kellersohn, Thomas; Korber, Nikolaus; Jansen, Martin. . Journal of the American Chemical Society (American Chemical Society (ACS)). 1993, 115 (24): 11254–11258. ISSN 0002-7863. doi:10.1021/ja00077a026.
- Schnick, Wolfgang; Jansen, Martin. Crystal structure of potassium and rubidium ozonide. Angewandte Chemie, 1985. 97 (1): 48-49. ISSN: 0044-8249
- A. W. Petrocelli and A. Capotosto. (报告). Washignton DC: National Aeronautics and Space Administration. November 1964. (原始内容存档于2021-11-08).
- Schnick, Wolfgang; Jansen, Martin. Preparation, crystal structure, and thermal behavior of potassium ozonide (PDF (页面存档备份,存于)). Revue de Chimie Minerale, 1987. 24 (4): 446-456. ISSN: 0035-1032
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.