花椒毒酚
花椒毒酚是一種呋喃香豆素。它是蛇床的主要有效成分之一。[3]它可由花椒毒素经三溴化硼脱甲基制得。[4]
| 花椒毒酚 | |
|---|---|
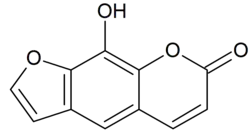 | |
| IUPAC名 9-Hydroxy-7H-furo[3,2-g][1]benzopyran-7-one | |
| 别名 | 8-Hydroxypsoralen 8-Hydroxypsoralene 8-Hydroxyfuranocoumarin |
| 识别 | |
| CAS号 | 2009-24-7 |
| PubChem | 65090 |
| ChemSpider | 58600 |
| SMILES |
|
| InChI |
|
| InChIKey | JWVYQQGERKEAHW-UHFFFAOYAF |
| 性质 | |
| 化学式 | C11H6O4 |
| 202.16 g/mol g·mol⁻¹ | |
| 熔点 | 240 °C(513 K)[1] 245 °C(518 K)[2] |
| 溶解性(水) | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
代謝
- 花椒毒酚-O-甲基轉移酶(8-羥基呋喃香豆素-8-O-甲基轉移酶)是一種酶。它會使用S-腺苷甲硫氨酸和花椒毒酚來生產S-腺苷高半胱氨酸和O-甲基花椒毒酚(花椒毒素或或甲氧沙林)。
參考文獻
- M. M. Abou-Elzahab, M. A. Metwally, A. M. Dawidar, M. Abdel-Mogib, E. A. Abu-Mustafa. . Bulletin of the Chemical Society of Japan. 1987-12, 60 (12): 4433–4435 [2021-11-23]. ISSN 0009-2673. doi:10.1246/bcsj.60.4433. (原始内容存档于2021-11-23) (英语).
- Lokar, Laura R. Coassini; Delben, Sergio. Taxonomical studies on Sesali elatum L. and allied species. Part 3. Photoactive furocoumarins in two populations of Seseli elatum. Phytochemistry, 1988. 27 (4): 1073-1077.
- Xanthotoxol exerts neuroprotective effects via suppression of the inflammatory response in a rat model of focal cerebral ischemia. He W, Chen W, Zhou Y, Tian Y and Liao F, Cell Mol Neurobiol., July 2013, volume 33, issue 5, pages 715-722, doi:10.1007/s10571-013-9939-2
- Bang-Le Zhang, Cheng-Qi Fan, Lei Dong, Fang-Dao Wang, Jian-Min Yue. . European Journal of Medicinal Chemistry. 2010-11, 45 (11): 5258–5264 [2021-11-23]. doi:10.1016/j.ejmech.2010.08.045. (原始内容存档于2022-06-24) (英语).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.