葡萄反应产物
葡萄反应产物(GRP、GRP1 或 2-S-谷硫酰基咖啡酰酒石酸)[1]是一种酚类化合物,可以解释为什么在加工过程中咖啡酰酒石酸会从葡萄汁中消失。[2]陈年红葡萄酒中也含有这种成分。[3]它由多酚氧化酶酶解产生,对限制葡萄汁的褐变非常重要,[4]特别是在白葡萄酒生产中。 该产品可在模型解决方案中再现。[5][6]
| 葡萄反应产物 | |
|---|---|
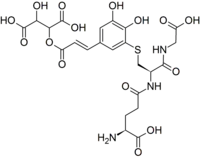 | |
| IUPAC名 2-[(E)-3-[3-[2-[(4-amino-4-carboxybutanoyl)amino]-3-(carboxymethylamino)-3-oxopropyl]sulfanyl-4,5-dihydroxyphenyl]prop-2-enoyl]oxy-3-hydroxybutanedioic acid | |
| 别名 | GRP GRP1 2-S-Glutathionyl caftaric acid 2-S-谷胱酰基咖啡酰酒石酸 |
| 识别 | |
| CAS号 | |
| PubChem | 71308212 |
| SMILES |
|
| ChEBI | 147433 |
| 性质 | |
| 化学式 | C23H27N3O15S |
| 摩尔质量 | 617.54 g·mol−1 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
通过质谱法可以确定其在葡萄酒中的浓度。[7]S-谷胱酰基咖啡酰酒石酸本身能被氧化。[8] 它不是葡萄多酚氧化酶的底物,但灰葡萄孢菌的漆酶可以利用它形成 GRP2。[9]
相关分子
其他相关分子是“反式”-咖啡酰酒石酸衍生物,如 GRP1,2-苯醌[10]和 2,5-di-S-谷氨酰基咖啡酰酒石酸盐(GRP2)[11]或与花青素的加合物。[12]

咖啡酒石酸紫外可见光谱
参考文献
- Veronique F. Cheynier; Eugene K. Trousdale; Vernon L. Singleton; Michel J. Salgues; Renee Wylde. . J. Agric. Food Chem. 1986, 34 (2): 217–221. doi:10.1021/jf00068a016.
- Caftaric acid in grapes and conversion to a reaction product during processing. V.L. Singleton, J. Zaya, E. Trousdale and M. Salgues, Vitis, 1984, pages 113-120 (article)
- Anis Arnous; Dimitris P. Makris; Panagiotis Kefalas. . J. Agric. Food Chem. 2001, 49 (12): 5736–5742. PMID 11743756. doi:10.1021/jf010827s.
- V.L. Singleton; M. Salgues; J. Zaya; E. Trousdale. (PDF). Am. J. Enol. Vitic. 1985, 36 (1): 50.
- V. Cheynier; C. Owe; J. Rigaud. . Journal of Food Science. 1988, 53 (6): 1729–1732. doi:10.1111/j.1365-2621.1988.tb07828.x.
- Veronique Cheynier; Jorge M. Ricardo da Silva. . J. Agric. Food Chem. 1991, 39 (6): 1047–1049. doi:10.1021/jf00006a008.
- Straightforward Method To Quantify GSH, GSSG, GRP, and Hydroxycinnamic Acids in Wines by UPLC-MRM-MS. Anna Vallverdú-Queralt, Arnaud Verbaere, Emmanuelle Meudec, Veronique Cheynier and Nicolas Sommerer, J. Agric. Food Chem. 2015, 63, 142−149, doi:10.1021/jf504383g
- Caftaric Acid Disappearance and Conversion to Products of Enzymic Oxidation in Grape Must and Wine. V. L. Singleton, M. Salgues, J. Zaya and E. Trousdale, Am. J. Enol. Vitic, 1985, volume 36, number 1, pages 50-56 'abstract)
- Salgues, M.; Cheynier, V.; Gunata, Z.; Wylde, R. . Journal of Food Science. 1986, 51 (5): 1191. doi:10.1111/j.1365-2621.1986.tb13081.x.
- Cheynier V.; Rigaud J.; Souquet J.-M.; Duprat F.; Moutounet M. . American Journal of Enology and Viticulture. 1990, 41 (4): 346–349.INIST:5402970
- Véronique Cheynier; Jacques Rigaud; Michel Moutounet. . Phytochemistry. 1990, 29 (6): 1751–1753. doi:10.1016/0031-9422(90)85008-4.
- Petros Kneknopoulos; George K. Skouroumounis; Yoji Hayasaka; Dennis K. Taylor. . J. Agric. Food Chem. 2011, 59 (3): 1005–1011. PMID 21214245. doi:10.1021/jf103682x.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.