1-氧杂-4-硫杂环己烷
1-氧杂-4-硫杂环己烷是一种有机化合物,化学式为C4H8OS。它可由2,2'-二氯乙醚(或2,2'-二溴乙醚)和硫氢化钾反应得到,[3]或由硫代二甘醇和丁基三氯化锡反应制得。[4]在催化下,它可以被过氧化氢氧化为亚砜或砜。[5]
| 1-氧杂-4-硫杂环己烷 | |
|---|---|
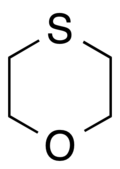 | |
| IUPAC名 1,4-Oxathiane | |
| 别名 | 1,4-Thioxane; p-Thioxane; 1-Oxa-4-thiacyclohexane |
| 识别 | |
| CAS号 | 15980-15-1 |
| 性质 | |
| 化学式 | C4H8OS |
| 摩尔质量 | 104.17 g·mol−1 |
| 熔点 | 30 °C(303 K)[1] |
| 沸点 | 147—149 °C(420—422 K)[2] |
| 危险性 | |
GHS危险性符号  | |
| GHS提示词 | Warning |
| H-术语 | H226, H315, H319, H335 |
| P-术语 | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338 |
| 致死量或浓度: | |
LD50(中位剂量) |
2830 mg/kg oral rat |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- James Ranald Alexander, Hamilton McCombie. . J. Chem. Soc. 1931, 0 (0): 1913–1918 [2022-05-30]. ISSN 0368-1769. doi:10.1039/JR9310001913 (英语).
- Frederick Richter, Frederick B. Augustine, Emil Koft, E. Emmet Reid. . Journal of the American Chemical Society. 1952-08, 74 (16): 4076–4079 [2022-05-30]. ISSN 0002-7863. doi:10.1021/ja01136a031. (原始内容存档于2022-05-30) (英语).
- Movsumzade, M. M.; Gurbanov, P. A.; Askerov, N. D.; Khodzhaev, G. Kh. Reaction of β,β'-dihalodialkyl ethers with potassium hydrosulfide(俄文). Azerbaidzhanskii Khimicheskii Zhurnal, 1979. 6: 86-90. ISSN 0005-2531.
- Giuseppe Tagliavini, Daniele Marton, Donatella Furlani. . Tetrahedron. 1989-01, 45 (4): 1187–1196 [2022-05-30]. doi:10.1016/0040-4020(89)80027-4. (原始内容存档于2022-07-09) (英语).
- Elena Badetti, Alessandro Bonetto, Francesco Romano, Luciano Marchiò, Cristiano Zonta, Giulia Licini. . Catalysis Letters. 2017-09, 147 (9): 2313–2318 [2022-05-30]. ISSN 1011-372X. doi:10.1007/s10562-017-2144-z (英语).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.