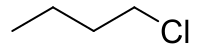
1-氯丁烷
1-氯丁烷,分子式C4H9Cl。
| 1-氯丁烷 | |
|---|---|
 | |
| IUPAC名 1-chlorobutane | |
| 别名 | 正丁基氯 氯丁烷 丁基氯 氯代正丁烷 |
| 识别 | |
| CAS号 | 109-69-3 |
| PubChem | 8005 |
| ChemSpider | 7714 |
| SMILES |
|
| InChI |
|
| InChIKey | VFWCMGCRMGJXDK-UHFFFAOYAR |
| 性质 | |
| 化学式 | C4H9Cl |
| 摩尔质量 | 92.57 g·mol−1 |
| 外观 | 无色有类似氯仿气味的挥发性液体 |
| 密度 | 0.88 g/mL |
| 熔点 | -123 °C |
| 沸点 | 79 °C |
| 溶解性(水) | 0.5 g/L (20 °C)[1] |
| 蒸氣壓 | 10.8 kPa (20 °C) |
| 折光度n D |
1.4018 (20 °C)[2] |
| 危险性 | |
| 警示术语 | R:R11 |
| 安全术语 | S:S2, S9, S16, S29 |
| MSDS | Oxford MSDS |
| 欧盟分类 | 可燃 (F) |
| NFPA 704 |
 3
1
1
|
| 致死量或浓度: | |
LD50(中位剂量) |
2670 mg·kg-1 (大鼠经口)[3] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
性质
无色挥发性易燃液体,有类似氯仿的气味。几乎不溶于水,与乙醇和乙醚混溶。
- 粘度:0.45 mPa·s(20°C)
- 闪点(开杯):-9.4 °C
- 自燃点:460 °C
- 与水的共沸物:水 6.6 wt%,共沸点 68.1 °C[4]
- 与醇的共沸物:[4]
- 甲醇 28.5 wt%,共沸点 57.2 °C
- 乙醇 21.5 wt%,共沸点 66.2 °C
- 正丙醇 16.0 wt%,共沸点 75.6 °C
- 蒸气压方程(安托万方程):
反應
参考资料
- Record of CAS RN 109-69-3 in the GESTIS Substance Database from the IFA
- Gerrard, W.; Hudson, H. R.; Murphy, W. S. "s-Butyl Chloride from n-Butyl Dichloroborinate and from n-Butanol-Hydrogen Chloride." J. Org. Chem. 1962, 1099–1101
- SICHERHEITSDATENBLATT
- Ullmann's Encyclopedia of Industrial Chemistry, Wiley Interscience, Release 2009, 7th Edition
- Kemme, H.R.; Kreps, S.I., Vapor Pressure of Primary n-Alkyl Chlorides and Alcohols in J. Chem. Eng. Data, 1969, 14, 1, 98-102.
- Stridth, G.; Sunner, S.: Enthalpies of formation of some 1-chloroalkanes and the CH2-increment in the 1-chloroalkanes series in J. Chem. Thermodyn., 1975, 7, 161-168.
- Grolier, J.-P.E.; Roux-Desgranges, G.; Berkane, M.; Jimenez, E.; Wilhelm, E.: Heat capacities and densities of mixtures of very polar substances 2. Mixtures containing N,N-dimethylformamide in J. Chem. Thermodynam., 1993, 25(1), 41-50.
- Majer, V.; Svoboda, V., Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation, Blackwell Scientific Publications, Oxford, 1985, 300
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.