10-甲氧基喜树碱
10-甲氧基喜树碱是一种有机化合物,分子式C21H18N2O5,是常见的喜树碱衍生物,具有抗肿瘤活性[2]。它可由10-羟基喜树碱和重氮甲烷在甲醇-二氧六环(1:1)中反应得到[3]。它在碘化亚铜-三乙胺催化下和氧气反应,可以得到5-羟基的衍生物[4]。

喜树碱的结构
| 10-甲氧基喜树碱 | |
|---|---|
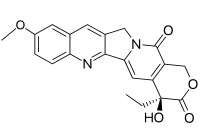 | |
| 识别 | |
| CAS号 | 19685-10-0 |
| SMILES |
|
| 性质 | |
| 化学式 | C21H18N2O5 |
| 摩尔质量 | 378.38 g·mol−1 |
| 外观 | 黄色固体[1] |
| 熔点 | 250.3~250.9 °C[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- Visible-Light-Induced Radical Cascade Cyclization: Synthesis of (20S)-Camptothecin, SN-38 and Irinotecan. Chinese Journal of Chemistry, 2018. doi:10.1002/cjoc.201800358
- Luo, P., He, Q., He, X., Hu, Y., Lu, W., Cheng, Y., & Yang, B. (2006). Potent antitumor activity of 10-methoxy-9-nitrocamptothecin. Molecular cancer therapeutics, 5(4), 962-968. doi:10.1158/1535-7163.MCT-05-0385
- Synthesis of new cytotoxic E-ring modified camptothecins. Tetrahedron Letters. 2010. doi:10.1016/j.tetlet.2010.09.130.
- Xungui He,Heyong Gao,Wei Lu & Junchao Cai. New Method for the Synthesis of 5‐Hydroxycamptothecin Derivatives. Synthetic Communications, 2004. doi:10.1081/SCC-200039356.
拓展阅读
- M Gao; K D. Miller; G W. Sledge; Q-H Zheng. Radiosynthesis of carbon-11-labeled camptothecin derivatives as potential positron emission tomography tracers for imaging of topoisomerase I in cancers. Bioorganic & Medicinal Chemistry Letters. 2005. 15 (17). doi:10.1016/j.bmcl.2005.05.108.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.