2,3-二甲基-1-丁烯
2,3-二甲基-1-丁烯是一种有机化合物,化学式为C6H12。它可由2,3-二甲基-1,3-丁二烯在催化剂的存在下进行加氢得到。[3]3,3-二甲基-2-丁醇和Burgess试剂反应虽然也会生成2,3-二甲基-1-丁烯,但是会同时产生较多的2,3-二甲基-2-丁烯与少量的3,3-二甲基-1-丁烯[4]。它进一步发生加氢反应,生成2,3-二甲基丁烷。[5]
| 2,3-二甲基-1-丁烯 | |
|---|---|
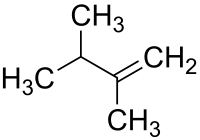 | |
| 识别 | |
| CAS号 | 563-78-0 |
| 性质 | |
| 化学式 | C6H12 |
| 摩尔质量 | 84.16 g·mol−1 |
| 外观 | 液体 |
| 密度 | 0.6732 g·cm−3[1] |
| 沸点 | 55.6 °C(328.8 K)[2] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- D.B. Brooks, F.L. Howard, H.C. Crafton. (PDF). Journal of Research of the National Bureau of Standards. 1940-01, 24 (1): 33 [2020-01-18]. ISSN 0091-0635. doi:10.6028/jres.024.018. (原始内容存档 (PDF)于2020-12-08) (英语).
- Robert G. Kelso, Kenneth W. Greenlee, John M. Derfer, Cecil E. Boord. . Journal of the American Chemical Society. 1952-01, 74 (2): 287–292 [2020-01-18]. ISSN 0002-7863. doi:10.1021/ja01122a001 (英语).
- Lutz Greb, Pascual Oña-Burgos, Birgitta Schirmer, Stefan Grimme, Douglas W. Stephan, Jan Paradies. . Angewandte Chemie International Edition. 2012-10-01, 51 (40): 10164–10168 [2020-01-18]. doi:10.1002/anie.201204007 (英语).
- Taibi, Pascale; Mobashery, Shahriar; Hart, Amy C. (Methoxycarbonylsulfamoyl)triethylammonium hydroxide. e-EROS Encyclopedia of Reagents for Organic Synthesis, 2008. pp 1-9. ISBN 978-0-470-84289-8.
- Laufenberg, Alfred; Behr, Arno; Keim, Wilhelm. Hydrogenation of aromatics, aldehydes, olefins, nitroalkanes, nitriles, and chlorohydrocarbons using metal salt catalysts in phase-transfer systems. 1989. DE 3841698 A1.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.