2-氯吡啶
2-氯吡啶是一种卤代烃,化学式为C5H4ClN。它是一种无色液体,主要用于工业生产杀菌剂和杀虫剂。它还可用于抗组胺药和抗心律失常药等药物生产。[2]
| 2-氯吡啶 | |||
|---|---|---|---|
 | |||
| |||
| IUPAC名 2-Chloropyridine | |||
| 英文名 | |||
| 别名 | 2-氯代吡啶 | ||
| 识别 | |||
| CAS号 | 109-09-1 | ||
| PubChem | 7977 | ||
| ChemSpider | 7689 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | OKDGRDCXVWSXDC-UHFFFAOYAI | ||
| UN编号 | 2822 | ||
| ChEBI | 39174 | ||
| RTECS | US5950000 | ||
| 性质 | |||
| 化学式 | C5H4ClN | ||
| 113.54 g·mol⁻¹ | |||
| 外观 | 无色液体 | ||
| 熔点 | -46 °C(227 K) | ||
| 沸点 | 166 °C(439 K) | ||
| 溶解性(水) | 27 g/L | ||
| pKa | 0.49 [1] | ||
| 危险性 | |||
GHS危险性符号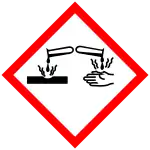    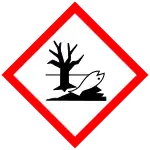 | |||
| GHS提示词 | 危险 | ||
| H-术语 | H301, H310, H315, H319, H330, H400 | ||
| P-术语 | P260, P261, P262, P264, P270, P271, P273, P280, P284, P301+310, P301+312, P302+350, P302+352, P304+340 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
制备
2-氯吡啶最初在1898年被报道,由2-羟基吡啶的氯化反应制得。[3]典型的氯化试剂是磷酰氯。2-氯吡啶也能通过吡啶的卤化来制得,反应生成2-氯代和2,6-二氯代产物的混合物。[2]
2-氯吡啶衍生物也能通过相应的吡啶-N-氧化物来制备。[4]
参考文献
- Linnell, R. H., J. Org. Chem., 1960, 25, 290.
- Shimizu, Shinkichi et al. Pyridine and Pyridine Derivatives. Ullmann’s Encyclopedia of Industrial Chemistry. 2000. doi:10.1002/14356007.a22_399
- Sell, William J.; Dootson, Frederick W. The chlorine derivatives of pyridine. Part I. Journal of the Chemical Society, Transactions 1898, 73, pp. 432-441. http://www.rsc.org/ejarchive/CT/1898/CT8987300432.pdf
- P. Naender, B. Gangadasu, Chilukuri Ramesh, B.C. Raju and V.J. Rao. Facile and Selective Synthesis of Chloromethypyridines and Chloropyridines using Diphosgene/Triphosgene. Synthetic Communications. 34, 6, 1097, 2004
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.