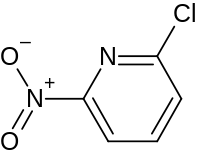
2-氯-5-硝基吡啶
2-氯-5-硝基吡啶是一种有机氯化合物,化学式为C5H3ClN2O2。它可由6-甲氧基-3-硝基吡啶和三氯氧磷在二甲基甲酰胺中反应制得;[2]或由2-氨基-5-硝基吡啶重氮化后和氯化亚铜反应得到。[3]它和苯硼酸在四(三苯基膦)钯催化下反应,可以得到5-硝基-2-苯基吡啶。[4]
| 2-氯-5-硝基吡啶 | |
|---|---|
 | |
| 识别 | |
| CAS号 | 4548-45-2 |
| PubChem | 78308 |
| SMILES |
|
| 性质 | |
| 化学式 | C5H3ClN2O2 |
| 摩尔质量 | 158.54 g·mol−1 |
| 外观 | 黄色固体[1] |
| 熔点 | 107.3-108.3 °C[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- One-Pot C−H Functionalization of Arenes by Diaryliodonium Salts. Angew Chem Int Ed. 2016. doi:10.1002/anie.201603175.
- Lai, Long-Li; Lin, Pen-Yuan; Wang, Jy-Shih; Hwu, Jih Ru; Shiao, Min-Jen; Tsay, Shwu-Chen. Substituent effect on the chlorination of 2-alkoxypyridines to give 2-chloropyridines under Vilsmeier-Haack conditions. Journal of Chemical Research, Synopses (1996), (4), 194-195.
- Deaminative chlorination of aminoheterocycles. Nature Chemistry (2022), 14(1), 78-84. doi:10.1038/s41557-021-00812-0.
- Olivier Lohse. The Palladium Catalysed Suzuki Coupling of 2- and 4-Chloropyridines. Synlett 1999; 1999(1): 45-48 doi:10.1055/s-1999-2533.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.