4-亚硝基二苯胺
4-亚硝基二苯胺是一种有机化合物,化学式为C12H10N2O。它可由4-亚硝基苯酚和苯胺在对甲苯磺酸存在下于甲醇中反应得到。[2]在钯催化下,它加氢得到N-苯基对苯二胺。[3]它和三异丁基铝反应,可以得到N-苯基-N'-异丁基对苯二胺。[4]
| 4-亚硝基二苯胺 | |
|---|---|
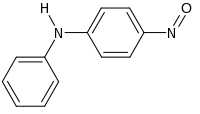 | |
| 识别 | |
| CAS号 | 156-10-5 |
| 性质 | |
| 化学式 | C12H10N2O |
| 摩尔质量 | 198.22 g·mol−1 |
| 熔点 | 143 °C(416 K)[1] |
| 沸点 | 292 °C(565 K)[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- "PhysProp" data were obtained from Syracuse Research Corporation of Syracuse, New York (US). Retrieved from SciFinder. [2022-12-06].
- John T. Hays, Herbert Lewis Young, Herbert H. Espy. . The Journal of Organic Chemistry. 1967-01, 32 (1): 158–162 [2022-12-05]. ISSN 0022-3263. doi:10.1021/jo01277a039. (原始内容存档于2022-12-05) (英语).
- Starovoitov, M. K.; Kachegin, A. F.; Rudakova, T. V.; Belousov, E. K.; Kozlov, A. I.; Safonov, S. A. Method for preparing 4-(phenylamino)aniline by the catalytic hydrogenation of 4-(phenylamino)nitrosobenzene. 2006 RU 2278108 C2.
- Zapevalov, G. A.; Kuz'min, S. V.; Kogan, L. M.; Krol, V. A. Products of the reaction of p-nitrosodiphenylamine with triisobutylaluminum. Doklady Akademii Nauk SSSR, 1984. 274 (2): 335-338. ISSN 0002-3264.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.