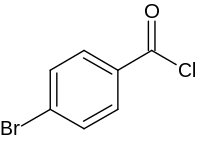
4-溴苯甲酰氯
4-溴苯甲酰氯是一种有机化合物,化学式为C7H4BrClO,它是苯甲酰氯苯环对位的氢被溴取代的化合物。它可由4-溴苯甲酸和草酰氯在二甲基甲酰胺的催化下于二氯甲烷中反应得到[1],酰氯化试剂也可使用氯化亚砜[2]。它和苯胺在三乙胺的存在下于四氢呋喃中反应,可以得到N-苯基-4-溴苯甲酰胺。[3]
| 4-溴苯甲酰氯 | |
|---|---|
 | |
| 别名 | 对溴苯甲酰氯 |
| 识别 | |
| CAS号 | 586-75-4 |
| PubChem | 68515 |
| 性质 | |
| 化学式 | C7H4BrClO |
| 摩尔质量 | 219.46 g·mol−1 |
| 熔点 | 42 °C(315 K) |
| 沸点 | 246 °C(519 K) |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- Kay Ahn, Markus Boehm, Matthew F. Brown, Jessica Calloway, Ye Che, Jinshan Chen, Kimberly F. Fennell, Kieran F. Geoghegan, Adam M. Gilbert, Jemy A. Gutierrez, Amit S. Kalgutkar, Adhiraj Lanba, Chris Limberakis, Thomas V. Magee, Inish O’Doherty, Robert Oliver, Brandon Pabst, Jayvardhan Pandit, Kevin Parris, Jeffrey A. Pfefferkorn, Timothy P. Rolph, Rushi Patel, Brandon Schuff, Veerabahu Shanmugasundaram, Jeremy T. Starr, Alison H. Varghese, Nicholas B. Vera, Cecile Vernochet, Jiangli Yan. . ACS Chemical Biology. 2016-09-16, 11 (9): 2529–2540 [2021-09-20]. ISSN 1554-8929. doi:10.1021/acschembio.6b00266 (英语).
- Hugo Gallardo, Rodrigo Cristiano, André Vieira, Ricardo Neves Filho, Rajendra Srivastava. . Synthesis. 2008-02, 2008 (4): 605–609 [2021-09-20]. ISSN 0039-7881. doi:10.1055/s-2008-1032156. (原始内容存档于2018-06-03) (英语).
- Clarice A. D. Caiuby, Matheus P. de Jesus, Antonio C. B. Burtoloso. . The Journal of Organic Chemistry. 2020-06-05, 85 (11): 7433–7445 [2021-09-20]. ISSN 0022-3263. doi:10.1021/acs.joc.0c00833. (原始内容存档于2021-11-07) (英语).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.