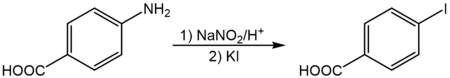
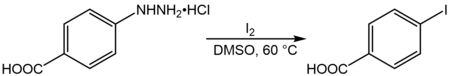
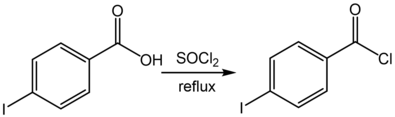
4-碘苯甲酸
4-碘苯甲酸是一种有机化合物,化学式为C7H5IO2,它是2-碘苯甲酸和3-碘苯甲酸的同分异构体。
| 4-碘苯甲酸 | |
|---|---|
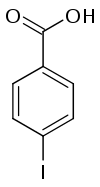 | |
 | |
| 英文名 | |
| 别名 | 对碘苯甲酸 |
| 识别 | |
| CAS号 | 619-58-9 |
| PubChem | 12085 |
| SMILES |
|
| InChIKey | GHICCUXQJBDNRN-UHFFFAOYSA-N |
| 性质 | |
| 化学式 | C7H5IO2 |
| 248.02 g·mol⁻¹ | |
| 外观 | 白色固体 |
| 密度 | 1.999±0.06 g·cm−3 |
| 熔点 | 269—270 °C(542—543 K)[1] |
| 溶解性 | 可溶于丙酮[2] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
结构及性质
4-碘苯甲酸和其它4-卤苯甲酸同构,属单斜晶系P21/n空间群,在固相以二聚体的形式存在。[2]
4-碘苯甲酸可以发生Suzuki反应,如和苯硼酸反应在钯催化剂的存在下,生成联苯-4-甲酸。[6]它也能发生羧酸的特征反应,如和甲醇反应,生成4-碘苯甲酸甲酯;[7]和氯化亚砜回流反应,得到4-碘苯甲酰氯。[8]
它可以和Mn2+、Co2+和Ni2+等金属离子形成配合物。[9]
参考文献
- Gorlushko, Dmitry A.; Filimonov, Victor D.; Krasnokutskaya, Elena A.; Semenischeva, Nadya I.; Go, Bong Seong; Hwang, Ho Yun; Cha, Eun Hye; Chi, Ki-Whan. . Tetrahedron Letters. 2008, 49 (6): 1080–1082. ISSN 0040-4039. doi:10.1016/j.tetlet.2007.11.192. Supplementary data (doc)
- Nygren, Cara L.; Wilson, Chick C.; Turner, John F. C. . The Journal of Physical Chemistry A. 2005, 109 (11): 2586–2593. ISSN 1089-5639. doi:10.1021/jp047189b.
- Dong, Chun-ping; Nakamura, Kentaro; Taniguchi, Toshihide; Mita, Soichiro; Kodama, Shintaro; Kawaguchi, Shin-ichi; Nomoto, Akihiro; Ogawa, Akiya; Mizuno, Takumi. . ACS Omega. 2018, 3 (8): 9814–9821. ISSN 2470-1343. doi:10.1021/acsomega.8b01559.
- Giri, Ramesh; Yu, Jin-Quan. Iodine monoacetate. e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd., 2008. pp 1-10. CODEN: 69KUHI. ISBN 978-0-470-84289-8
- Frank C. Whitmore and Gladys E. Woodward. p-IODOBENZOIC ACID [Benzoic acid, p-iodo-] (页面存档备份,存于). Org. Synth. 1927, 7, 58. doi:10.15227/orgsyn.007.0058
- Jebali, Zayneb; Granados, Albert; Nabili, Abdelkader; Boufi, Sami; do Rego, Ana Maria B.; Majdoub, Hatem; Vallribera, Adelina. . Cellulose. 2018, 25 (12): 6963–6975. ISSN 0969-0239. doi:10.1007/s10570-018-2085-8.
- Chisholm, David R.; Tomlinson, Charles W. E.; Zhou, Garr-Layy; Holden, Claire; Affleck, Valerie; Lamb, Rebecca; Newling, Katherine; Ashton, Peter; Valentine, Roy; Redfern, Christopher; Erostyák, János; Makkai, Geza; Ambler, Carrie A.; Whiting, Andrew; Pohl, Ehmke. . ACS Chemical Biology. 2019, 14 (3): 369–377. ISSN 1554-8929. doi:10.1021/acschembio.8b00916.
- Wang, Qun; Liu, Long; Dong, Jianyu; Tian, Zhibin; Chen, Tieqiao. . New Journal of Chemistry. 2019, 43 (24): 9384–9388. ISSN 1144-0546. doi:10.1039/C9NJ01748H.
- Churagov, F. M.; Gambarov, D. G.; Mamedov, Kh. S. Several properties and structure of manganese(II), cobalt(II) and nickel(II) complexes with p-derivatives of benzoic acid. Koordinatsionnaya Khimiya, 1991. 17 (10): 1404-1411. ISSN: 0132-344X.
拓展阅读
- Grineva, Olga V.; Zorky, Petr M.; Rostov, Evgenij S. . Structural Chemistry. 2007, 18 (4): 443–448. ISSN 1040-0400. doi:10.1007/s11224-007-9161-2.
- Lewandowski, W.; Barańska, H.; Mościbroda, P.; Dasiewicz, B. . Journal of Molecular Structure. 1993, 293: 89–92. ISSN 0022-2860. doi:10.1016/0022-2860(93)80022-N.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.