4-(二甲基氨基)苯基五唑
4-(二甲基氨基)苯基五唑,是一个不稳定的、有爆炸性的化合物,它包含了非常罕见的由五个氮原子组成的五唑环。苯环对位上的二甲基氨基的给电子效应使得此化合物比苯基五唑更稳定。在室温下,其化学半衰期只有几个小时,然而在超低温下储存是可能的。这个化合物在1956年[1][2][3]与其他取代的芳基五唑一起被初次制备出来。对各种其他的衍生物的研究也在进行,但必定由于这些化合物的不稳定性而受到限制。[4][5][6][7][8] 一些取代基更多的衍生物,如 2,6-二羟基-4-(二甲基氨基)苯基五唑,要稍微更稳定一些,但是反过来,制备这些衍生物的难度更大。[9][10]目前的研究聚焦于形成这些五唑衍生物的过渡金属配合物,因为五唑环可能由于与金属中心的化学键而稳定下来。[11][12][13]
| 4-(二甲基氨基)苯基五唑 | |
|---|---|
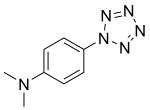 | |
| IUPAC名 4-(二甲基氨基)苯基五唑 | |
| 识别 | |
| CAS号 | 58402-54-3 |
| PubChem | 23279314 |
| ChemSpider | 10447638 |
| SMILES |
|
| 性质 | |
| 化学式 | C8H10N6 |
| 190.205 g·mol⁻¹ | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- Huisgen R, I. Ugi. Zur Losung eines klassichen Problems der organischen Stickstoff-Chemie. Angewandte Chemie. 1956; 68:705-706.
- Ugi I, R. Huisgen. Pentazole II. Die Zerfallsgeschwindigkeit der Arylpentazole. Chemische Berichte. 1958; 91:531-537.
- Ugi I, Perlinger H, Perlinger L. Pentazole III. Kristallisierte Aryl-pentazole. Chemische Berichte 1958; 98:2324-2329,
- John D. Wallis and Jack D. Dunitz. An all-nitrogen aromatic ring system: structural study of 4-dimethyl-aminophenylpentazole. Journal of the Chemical Society. Chemical Communications. 1983: 910-911.
- Butler, Richard N.; Collier, Seamus; Fleming, Adrienne F. M. . Journal of the Chemical Society, Perkin Transactions 2 (Royal Society of Chemistry (RSC)). 1996, (5): 801. ISSN 0300-9580. doi:10.1039/p29960000801.
- Butler, Richard N.; Fox, Anthony; Collier, Seamus; Burke, Luke A. . Journal of the Chemical Society, Perkin Transactions 2 (Royal Society of Chemistry (RSC)). 1998, (10): 2243–2248. ISSN 0300-9580. doi:10.1039/a804040k.
- Benin V, Kaszynski P, Radziszewski JG. . The Journal of Organic Chemistry. February 2002, 67 (4): 1354–8. PMID 11846686. doi:10.1021/jo0110754.
- Carlqvist, Peter; Östmark, Henrik; Brinck, Tore. . The Journal of Physical Chemistry A (American Chemical Society (ACS)). 2004, 108 (36): 7463–7467. ISSN 1089-5639. doi:10.1021/jp0484480.
- Efforts to synthesize the pentazolate anion
- David Adam. The synthesis and characterisation of halogen and nitro phenyl azide derivatives as highly energetic materials. PhD dissertation, Ludwig-Maximilans-Universität München, 2001 (页面存档备份,存于)
- Tsipis AC, Chaviara AT. . Inorganic Chemistry. February 2004, 43 (4): 1273–86. PMID 14966962. doi:10.1021/ic035112g.
- Burke, Luke A.; Fazen, Paul J. . Chemical Communications (Royal Society of Chemistry (RSC)). 2004, (9): 1082. ISSN 1359-7345. doi:10.1039/b315812h.
- Burke, Luke A.; Fazen, Paul J. . International Journal of Quantum Chemistry (Wiley-Blackwell). 2009, 109 (15): 3613–3618. ISSN 0020-7608. doi:10.1002/qua.22408.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.