5-氨基-1,3,4-噻二唑-2-硫酮
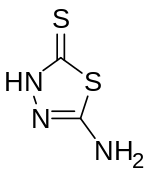
5-氨基-1,3,4-噻二唑-2-硫酮是一种有机化合物,化学式为C2H3N3S2。它可由氨基硫脲和二硫化碳在DMF中回流[2]或碳酸钠存在下于乙醇中回流[3]反应得到。它和异氰酸苯酯反应,可以得到N-苯基-N'-(5-巯基-1,3,4-噻二唑-2-基)脲。[4]它和丙炔酸甲酯在水中加热反应,可以得到3-(5-氨基-1,3,4-噻二唑-2-硫基)丙烯酸甲酯。[5]
| 5-氨基-1,3,4-噻二唑-2-硫酮 | |
|---|---|
 | |
| 别名 | 2-氨基-5-巯基-1,3,4-噻二唑 |
| 识别 | |
| CAS号 | 2349-67-9 |
| 性质 | |
| 化学式 | C2H3N3S2 |
| 摩尔质量 | 133.2 g·mol−1 |
| 外观 | 黄色晶体[1] |
| 熔点 | 236—238 °C(509—511 K)(分解)[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- S. Talath, A.K. Gadad. . European Journal of Medicinal Chemistry. 2006-08, 41 (8): 918–924 [2023-02-27]. doi:10.1016/j.ejmech.2006.03.027. (原始内容存档于2023-03-15) (英语).
- You-Ming Zhang, Ming-Xia Liu, Qi Lin, Qiao Li, Tai-Bao Wei. . Journal of Chemical Research. 2010-11, 34 (11): 619–621 [2023-02-27]. ISSN 1747-5198. doi:10.3184/030823410X12876712319282. (原始内容存档于2023-02-26) (英语).
- Vaibhav Dubey, Manish Pathak, Hans R. Bhat, Udaya P. Singh. . Chemical Biology & Drug Design. 2012-10, 80 (4): 598–604 [2023-02-27]. doi:10.1111/j.1747-0285.2012.01433.x. (原始内容存档于2023-02-26) (英语).
- Mohammad M. Al-Sanea, Abdelrahman Hamdi, Simone Brogi, Samar S. Tawfik, Dina I. A. Othman, Mahmoud Elshal, Hidayat Ur Rahman, Della G. T. Parambi, Rehab M. Elbargisy, Samy Selim, Ehab M. Mostafa, Ahmed A. B. Mohamed. . Journal of Enzyme Inhibition and Medicinal Chemistry. 2023-12-31, 38 (1) [2023-02-27]. ISSN 1475-6366. doi:10.1080/14756366.2022.2162511. (原始内容存档于2023-02-26) (英语).
- Haiying Wang, Jiewei Rong. . HETEROCYCLES. 2022, 104 (12) [2023-02-27]. ISSN 0385-5414. doi:10.3987/COM-22-14736. (原始内容存档于2023-02-26) (英语).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.