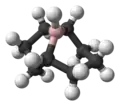
9-硼二环[3.3.1]壬烷
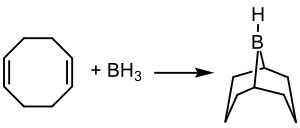
9-硼二环[3.3.1]壬烷(9-BBN)是一个双环有机硼化合物,由1,5-环辛二烯与乙硼烷-二甲基硫醚加合物(甲硼烷)在二甲氧基乙烷中作用制得:[1]
 |
 |
| 9-硼二环[3.3.1]壬烷 | |||
|---|---|---|---|
| |||
 | |||
 | |||
| IUPAC名 9-Borabicyclo[3.3.1]nonane | |||
| 别名 | 9-硼杂双环-[3.3.1]壬烷 | ||
| 识别 | |||
| 缩写 | 9-BBN | ||
| CAS号 | 280-64-8 | ||
| PubChem | 6327450 | ||
| ChemSpider | 71299 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | FEJUGLKDZJDVFY-UHFFFAOYAE | ||
| EINECS | 206-000-9 | ||
| 性质 | |||
| 化学式 | C8H15B | ||
| 122.02 g·mol⁻¹ | |||
| 密度 | 0.894 g/cm3 | ||
| 熔点 | 153-155 °C(固态二聚体) | ||
| 溶解性(水) | 反应 | ||
| 危险性 | |||
| 警示术语 | R:R11, R14/15, R36/37/38 | ||
| 安全术语 | S:S7/9, S16, S33, S7/8, S26, S37/39 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
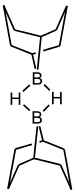
它一般以固态或溶于THF的形式出售,固态时为氢桥连的二聚体,在空气中较稳定。
9-BBN可作硼氢化反应试剂,与烯烃反应有较高的选择性,控制条件时,可以只与位阻较小的双键反应。生成的烷基硼可以进行多种反应,如发生氧化得到醇,发生还原得到烃,以及发生Suzuki反应等。[2]
参考资料
- Simple, remarkably efficient route to high purity, crystalline 9-borabicyclo[3.3.1]nonane (9-BBN) dimer John A. Soderquist, Herbert C. Brown; J. Org. Chem.; 1981; 46(22); 4599-4600. Abstract (页面存档备份,存于)
- Tatsuo Ishiyama, Norio Miyaura, and Akira Suzuki "Palladium(0)-catalyzed reaction of 9-alkyl-9-borabicyclo[3.3.1]nonane with 1-bromo-1-phenylthioethene: 4-(3-cyclohexenyl)-2-phenylthio-1-butene." Organic Syntheses, Coll. Vol. 9, p.107; Vol. 71, p.89 Article (页面存档备份,存于)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.