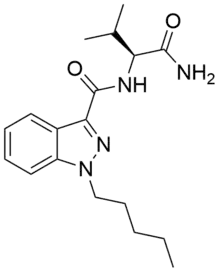
AB-PINACA
AB-PINACA是一种有机化合物,化学式为C18H26N4O2。它最初由辉瑞公司于2009年作为镇痛药开发,[1][2]并在2012年于日本首次被鉴定为合成大麻素。[3]
 | |
| 法律規範狀態 | |
|---|---|
| 法律規範 |
|
| 识别 | |
| |
| CAS号 | 1445752-09-9 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| 化学 | |
| 化学式 | C18H26N4O2 |
| 摩尔质量 | 330.43 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
它是潜在的CB1受体(Ki = 2.87 nM,EC50 = 1.2 nM)和CB2受体(Ki = 0.88 nM,EC50 = 2.5 nM)的激动剂。[4][5]
这种合成大麻素有不少相关死亡和住院病例报告。[6]
参见
- 5F-AB-PINACA
- 5F-ADB
- 5F-AMB
- 5F-APINACA
- 5F-CUMYL-PINACA
- AB-CHFUPYCA
- AB-FUBINACA
- AB-PICA
- ADB-CHMINACA
- ADB-FUBINACA
- ADB-PINACA
- ADBICA
- APICA
- APINACA
- MDMB-CHMICA
- PX-3
参考文献
- . Cayman Chemical. [25 June 2015]. (原始内容存档于2015-06-26).
- . [2023-01-26]. (原始内容存档于2016-05-27).
- Uchiyama N, Matsuda S, Wakana D, Kikura-Hanajiri R, Goda Y. . Forensic Toxicology. 2012, 31: 93–100. S2CID 25242453. doi:10.1007/s11419-012-0171-4.
- Banister SD, Moir M, Stuart J, Kevin RC, Wood KE, Longworth M, et al. . ACS Chemical Neuroscience. September 2015, 6 (9): 1546–59. PMID 26134475. doi:10.1021/acschemneuro.5b00112.
- Wiley JL, Marusich JA, Lefever TW, Antonazzo KR, Wallgren MT, Cortes RA, et al. . The Journal of Pharmacology and Experimental Therapeutics. September 2015, 354 (3): 328–39 [2023-01-26]. PMC 4538877
 . PMID 26105953. doi:10.1124/jpet.115.225326. (原始内容存档于2023-01-30).
. PMID 26105953. doi:10.1124/jpet.115.225326. (原始内容存档于2023-01-30). - Trecki J, Gerona RR, Schwartz MD. . The New England Journal of Medicine. July 2015, 373 (2): 103–7. PMID 26154784. doi:10.1056/NEJMp1505328.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.