Danheiser成环反应
Danheiser成环反应(Danheiser annulation),又称Danheiser TMS-环戊烯成环反应(Danheiser TMS-Cyclopentene annulation)[1][2][3][4][5][6]
α,β-不饱和酮与三烃基硅基(如三甲基硅基、三异丙基硅基)丙二烯在路易斯酸存在下发生反应,生成三烃基硅基环戊烯。
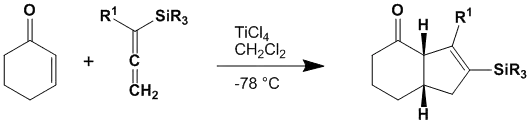
Danheiser成环反应
α,β-不饱和醛太活泼,反应会生成大量副产物;而α,β-不饱和酯则太不活泼,反应速度慢,产率低;环状和开链的酮都可以用作反应底物。这个反应是同面反应,具有高度立体选择性。
反应机理
路易斯酸四氯化钛首先活化α,β-不饱和酮的羰基,得到烯丙位碳正离子中间体,然后被三烃基硅基丙二烯进攻得到乙烯基碳正离子中间体A;临近的硅原子可以稳定乙烯基碳正离子(中间体B),或者可以发生1,2-重排反应得到中间体C;接下来钛烯醇化物进攻中间体C的碳正离子就可以得到环戊烯。有时中间体A也会生成副产物D–F。
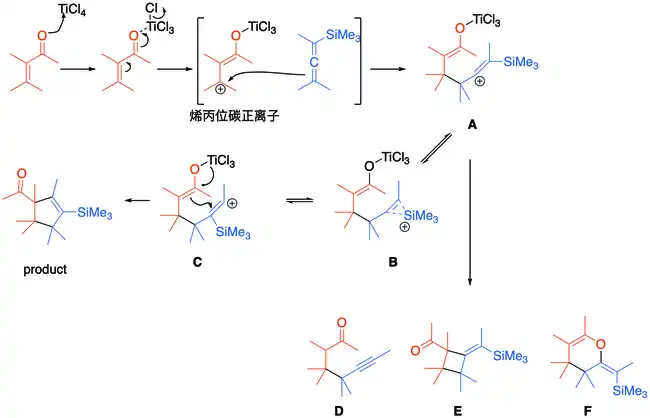
Danheiser成环反应机理
参见
参考资料
- "TMS-Cyclopentene Annulation: A Regiocontrolled Approach to the Synthesis of Five-Membered Rings", R. L. Danheiser, D. J. Carini, and A. Basak, J. Am. Chem. Soc. 1981, 103, 1604.
- "Scope and Stereochemical Course of the (Trimethylsilyl)cyclopentene Annulation", R. L. Danheiser, D. J. Carini, D. M. Fink, and A. Basak, Tetrahedron 1983, 39, 935.
- "The Reaction of Allenysilanes with α,β-Unsaturated Acylsilanes: New Annulation Approaches to Five and Six-Membered Carbocyclic Compounds", R. L. Danheiser and D. M. Fink, Tetrahedron Lett. 1985, 26, 2513.
- "A General [3+2] Annulation: cis-4-Exo-isopropenyl-1,9-dimethyl-8-(trimethylsilyl)bicyclo-[4.3.0]non-8-en-2-one", R. L. Danheiser, D. M. Fink, and Y. -M. Tsai, Organic Syntheses 1988, 66, 8.
- "Organic Syntheses Based on Name Reactions", By Alfred Hassner and C. Stumer, Elsevier, p. 78, 2002
- "Name Reactions: A Collection of Detailed Reaction Mechanisms", By Jie Jack Li, Springer, p. 102, 2003
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.