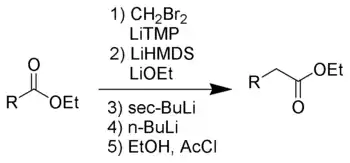
科瓦尔斯基酯增碳反应
反应机理
此反应的具体机理仍有争议。有人提出如下机理:
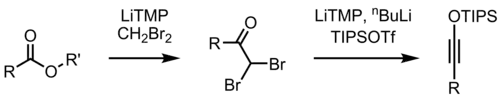
科瓦尔斯基酯增碳反应的可能机理
亦有认为,反应历程首先为锂化合物对酯羰基的加成,然后发生消除,生成二溴烯醇锂盐,再锂化,并发生α-消除得到烯基卡宾、发生烷基迁移,从而最后醇解,得酯。图
参考资料
- Conrad J. Kowalski, M. Serajul Haque, Kevin W. Fields. . J. Am. Chem. Soc. 1985, 107 (5): 1429–1430. doi:10.1021/ja00291a063.
- Rajarathnam E. Reddy and Conrad J. Kowalski, "Ethyl 1-Naphthylacetate: Ester Homologation via Ynolate Anions (页面存档备份,存于)", Organic Syntheses, Coll. Vol. 9, p.426 (1998); Vol. 71, p.146 (1993).
- Amos B. Smith, III, Sergey A. Kozmin, Christopher M. Adams, and Daniel V. Paone. . J. Am. Chem. Soc. 2000, 122 (20): 4984–4985. doi:10.1021/ja000430p.
- Conrad J. Kowalski, G. Sankar. Lal, M. Serajul. Haque. . J. Am. Chem. Soc. 1986, 108 (22): 7127–7128. doi:10.1021/ja00282a061.
- Diane Gray, Carmen Concellón, and Timothy Gallagher. . J. Org. Chem. 2004, 69 (14): 4849–4851. doi:10.1021/jo049562h.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.