 | |
 | |
| Names | |
|---|---|
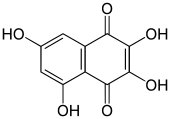
| Preferred IUPAC name
2,3,5,7-Tetrahydroxynaphthalene-1,4-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H6O6 | |
| Molar mass | 222.15 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2,3,5,7-Tetraahydroxy-1,4-naphthalenedione, also called 2,3,5,7-tetrahydroxynaphthoquinone or spinochrome B, is an organic compound with formula C
10H
6O
4, formally derived from 1,4-naphthoquinone through the replacement of four hydrogen atoms by hydroxyl (OH) groups.
Spinochrome B occurs naturally as pigment in the shell and spines of sea urchins such as the Japanese dull red species aka-uni (Pseudocentrotus depressus), the greenish-black murasaki-uni (Heliocidaris crassispina), and the brown bafun-uni (Strongylocentrotus pulcherrimus).[1][2] It is soluble in methanol and crystallizes as bright red needles that sublime above 200 °C.[2]
The compound gives a greenish yellow solution when treated with sodium hydroxide, a green solution with ferric chloride, and a green precipitate with lead acetate. It forms a fourfold acetate ester, C
10H
2O
2(CH
3COO)4, that crystallizes from methanol as yellow needles that melt at 157 °C.[2]
See also
- Hexahydroxynaphthoquinone (spinochrome E)
- 2,3,5,6,8-Pentahydroxy-1,4-naphthalenedione (spinochrome D)
References
- ↑ Chika KURODA and Masae OKAJIMA (1958), Studies on the Derivatives of Naphthoquinones, XIV. The pigments of sea urchins, IX. Proc. Japan Acad., volume 34, pages 616--618. Online version accessed on 2010-02-01. Pigment M2 is drawn as 2,3,6,8-thNQ but that is the same as 2,3,5,7-thNQ. The genus Heliocedaris should be Heliocidaris and the species purcherrmus should be pulcherrimus.
- 1 2 3 Chika KURODA and Masae OKAJIMA (1967), Studies on the Derivatives of Naphthoquinones, XVIII. The pigments of sea urchins, XIII. Proc. Japan Acad., volume 43, pages 41--44. Online version accessed on 2010-02-01.