 | |
| Names | |
|---|---|
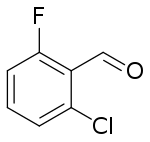
| IUPAC name
2-chloro-6-fluorobenzaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.237 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H4ClFO | |
| Molar mass | 158.56 g·mol−1 |
| Appearance | White solid |
| Melting point | 32–35 °C (89.6-95 °F; 305–308 K) |
| Boiling point | 104–105 °C (219–221 °F; 377–378 K) |
| Insoluble in water | |
| Solubility | Soluble in methanol, ethanol |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335, H401 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P321, P362+P364, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 101 °C (214 °F; 374 K) |
| Related compounds | |
Related compounds |
2-Chloro-6-fluorobenzaldoxime Chlorobenzaldehyde Fluorobenzaldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2-Chloro-6-fluorobenzaldehyde is a halogenated benzaldehyde derivative with the formula C6H3ClFCHO. It is an intermediate in the synthesis of other halogenated heterocyclic compounds.[1][2]
Uses and preparation
2-Chloro-6-fluorobenzaldehyde is a synthetic intermediate in the production of dicloxacillin and flucloxacillin. In addition it is used in the production of pesticides.
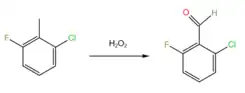
2-Chloro-6-fluorobenzaldehyde is prepared by oxidation of 2-chloro-6-fluorotolulene by hydrogen peroxide.[3]
References
- ↑ Daniewski, Andrzej R.; Liu, Wen; Püntener, Kurt; Scalone, Michelangelo (2002-05-01). "Two Efficient Methods for the Preparation of 2-Chloro-6-methylbenzoic Acid". Organic Process Research & Development. 6 (3): 220–224. doi:10.1021/op0102363. ISSN 1083-6160.
- ↑ Naveen, S.; Kavitha, Chandagirikoppal V.; Sarala, G.; Anandalwar, SridharM.; Prasad, J. Shashidhara; Rangappa, Kanchugarakoppal S. (2006). "Crystal Structure of 3-(2-Chloro-6-fluorophenyl)-2-(4-methoxyphenyl)-acrylonitrile". Analytical Sciences: X-ray Structure Analysis Online. 22: X291–X292. doi:10.2116/analscix.22.x291. ISSN 1348-2238.
- ↑ Bunnett, J. F.; Miles, J. H.; Nahabedian, K. V. (1961). "Kinetics and Mechanism of the Alkali Cleavage of 2,6-Dihalobenzaldehydes 1". Journal of the American Chemical Society. 83 (11): 2512–2516. doi:10.1021/ja01472a022. ISSN 0002-7863.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
