Lanthanide trichlorides are a family of inorganic compound with the formula LnCl3, where Ln stands for a lanthanide metal. The trichlorides are standard reagents in applied and academic chemistry of the lanthanides. They exist as anhydrous solids and as hydrates.
Properties
The anhydrous solids have melting points range from ca. 582 (Tb) - 925 °C (Lu). They are generally pale colored, often white. As coordination polymers, they only dissolve in donor solvents, including water.
| MCl3 | color | structure type | f-configuration | comment |
|---|---|---|---|---|
| ScCl3 | colorless | AlCl3-type | f0 | not classified as a lanthanide usually |
| YCl3 | colorless | AlCl3-type | f0 | not classified as a lanthanide usually |
| LaCl3 | colorless | UCl3-type | f0 | diamagnetic |
| CeCl3 | colorless | UCl3-type | f1, doublet | - |
| PrCl3 | green | UCl3-type | f2, triplet | - |
| NdCl3 | pink | UCl3-type | f3, quartet | - |
| PmCl3 | green | UCl3-type | f4, quintet | radioactive |
| SmCl3 | yellow | UCl3-type | f5, sextet | - |
| EuCl3 | yellow | UCl3-type | f6, septet | - |
| GdCl3 | colorless | UCl3-type | f7, octet | symmetrical electronic shell |
| TbCl3 | white | PuBr3-type | f8, septet | - |
| DyCl3 | white | AlCl3-type | f9, sextet | - |
| HoCl3 | yellow | AlCl3-type | f10, quintet | - |
| ErCl3 | violet | AlCl3-type | f11, quartet | - |
| TmCl3 | yellow | AlCl3-type | f12, triplet | - |
| YbCl3 | colorless | YCl3-type | f13, doublet | - |
| LuCl3 | colorless | AlCl3-type | f14 | diamagnetic |
Preparation
The lanthanide oxides and carbonates dissolve in hydrochloric acid to give chloride salt of the hydrated cations:
- M2O3 + 6 HCl + n H2O → 2 [Ln(H2O)n]Cl3
Industrial routes
Anhydrous trichlorides are produced commercially by carbothermic reaction of the oxide:[2]
- M2O3 + 3 Cl2 + 3 C → 2 MCl3 + 3 CO
Ammonium chloride route
The ammonium chloride route refers to a general procedure to produce anhydrous lanthanide chlorides. The method has the advantages of being general for the 14 lanthanides and it produces air-stable intermediates that resist hydrolysis. The use of ammonium chloride as a reagent is convenient because the salt is anhydrous, even when handled in air. Ammonium chloride is also attractive because it thermally decomposes to volatile products at temperatures compatible with the stability of the trichloride targets.[3][4][5]
- Step 1
- preparation of ammonium lanthanide chlorides
The reaction of an intimate mixture of lanthanide oxides with excess ammonium chloride produces anhydrous ammonium salts of the penta- and hexachlorides. Typical reaction conditions are hours at 230-250 °C.[4] Some lanthanides (as well as scandium and yttrium) form pentachlorides:
- M2O3 + 10 NH4Cl → 2 (NH4)2MCl5 + 3 H2O + 6 NH3
(M = Dy, Ho, Er, Tm, Lu, Yb, Y, Sc)
- Tb4O7 + 22 NH4Cl → 4 (NH4)2TbCl5 + 7 H2O + 14 NH3
Other lanthanides for hexachlorides:
- M2O3 + 12 NH4Cl → 2 (NH4)3MCl6 + 3 H2O + 6 NH3
(M = La, Ce, Nd, Pm, Sm, Eu, Gd)
- Pr6O11 + 40 NH4Cl → 6 (NH4)3PrCl6 + 11 H2O + 22 NH3
These reactions can also start with the metals, e.g.:[4]
- Y + 5 NH4Cl → (NH4)2YCl5 + 1.5 H2 + 3 NH3
- Step 2
- thermolysis of ammonium lanthanide chlorides
The ammonium lanthanum chlorides are converted to the trichlorides by heating in a vacuum. Typical reaction temperatures are 350–400 °C:[4]
- (NH4)2MCl5 → MCl3 + 2 HCl + 2 NH3
- (NH4)3MCl6 → MCl3 + 3 HCl + 3 NH3
Other methods
Hydrated lanthanide trichlorides dehydrate under a hot stream of hydrogen chloride.[3]
Structures
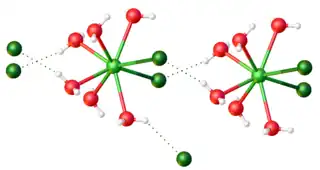
As indicated in the table, the anhydrous trichlorides follow two main motifs, UCl3 and YCl3. The UCl3 structure features 9-coordinate metal centers. The PuBr3 structure, adopted uniquely by TbCl3, features 8-coordinated metals. The remaining later metals are 6-coordinate as is aluminium trichloride.[7]
Reactions
Lanthanide trichlorides are commercial precursors to the metals by reduction, e.g. with aluminium:[2]
- LnCl3 + Al → Ln + AlCl3
In some cases, the trifluoride is preferred.
They react with humid air to give oxychlorides:
- LnCl3 + H2O → LnOCl + 2 HCl
For synthetic chemists, this reaction is a problematic since the oxychlorides are less reactive.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- 1 2 I. McGill (2005). "Rare Earth Elements". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_607. ISBN 978-3527306732.
- 1 2 Brauer, G., ed. (1963). Handbook of Preparative Inorganic Chemistry (2nd ed.). New York: Academic Press.
- 1 2 3 4 Meyer, G. (1989). "The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides—The Example of Ycl 3". The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides-The Example of YCl3. Inorganic Syntheses. Vol. 25. pp. 146–150. doi:10.1002/9780470132562.ch35. ISBN 978-0-470-13256-2.
- ↑ Edelmann, F. T.; Poremba, P. (1997). Herrmann, W. A. (ed.). Synthetic Methods of Organometallic and Inorganic Chemistry. Vol. VI. Stuttgart: Georg Thieme Verlag. ISBN 978-3-13-103021-4.
- ↑ Habenschuss, A.; Spedding, F. H. (1980). "Dichlorohexaaquagadolinium(III) Chloride (GdCl2(H2O)6)C". Crystal Structure Communications. 9: 213-218.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Cotton, Simon A. (2011). "Scandium, Yttrium & the Lanthanides: Inorganic & Coordination Chemistry". Encyclopedia of Inorganic and Bioinorganic Chemistry. doi:10.1002/9781119951438.eibc0195. ISBN 9781119951438.