| Autoimmune enteropathy | |
|---|---|
| Other names | Severe immune-mediated enteropathy, Immune-mediated protracted diarrhea of infancy |
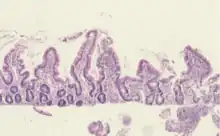 | |
| Histological evidence of enteropathy (inflammatory infiltrate, villus blunting) seen in this intestinal biopsy from a child with malnutrition. | |
| Specialty | Immunology Gastroenterology |
| Symptoms | Diarrhea, and autoimmune damage to the intestinal mucosa.[1] |
| Complications | Electrolyte imbalances, malabsorption, and failure to thrive.[2] |
| Usual onset | First six months of life.[3] |
| Duration | Chronic.[4] |
| Diagnostic method | histological changes, serologic testing, and clinical signs and symptoms.[5] |
| Differential diagnosis | Graft-versus-host disease, Crohn's disease, celiac disease and lactose intolerance.[6] |
| Treatment | Parenteral nutrition and corticosteroids.[7] |
| Prognosis | 30% mortality rate without treatment.[8] |
| Frequency | <1 in 100,000 infants.[1] |
Autoimmune enteropathy is a rare autoimmune disorder characterized by weight loss from malabsorption, severe and protracted diarrhea, and autoimmune damage to the intestinal mucosa.[1] Autoimmune enteropathy typically occurs in infants and younger children however, adult cases have been reported in literature.[9] Autoimmune enteropathy was first described by Walker-Smith et al. in 1982.[10]
The mechanisms of autoimmune enteropathy isn't well known but dysfunction or deficiency of CD25+CD4+ regulatory T cells may play a role.[11] Numerous other illnesses and syndromes are linked to autoimmune enteropathy, the most prominent being Autoimmune polyendocrine syndrome type 1 and immune dysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome.[5]
Clinical symptoms, laboratory results, and the histological characteristics of a small bowel biopsy are used to make the diagnosis.[5] These patients typically don't respond to diet modification and often require immune-suppressants and sometimes require total parenteral nutrition.[12] The prevalence of autoimmune enteropathy is estimated to be less than 1 in 100,000 infants[1]
The prognosis of autoimmune enteropathy varies and depends on systemic manifestations, the severity of symptoms, and the degree of gastrointestinal involvement.[13] Children suffering from autoimmune enteropathy are frequently vulnerable to systemic and local infections pertaining to immunotherapy, the intestinal and skin barriers, and malnourishment.[14]
Signs and symptoms
Autoimmune enteropathy usually presents within the first six months of life. Symptoms are typically seen by two to four weeks of age.[3] The hallmark feature of autoimmune enteropathy is severe high-output diarrhea. As a result, patients may develop significant electrolyte abnormalities, malabsorption, and growth failure.[2] More than 60% of cases have an estimated average stool output that is extremely high upon the time of diagnosis.[15]
Multisystem manifestations may include renal, endocrine,[7] hematologic,[16][17] musculoskeletal system, pulmonary,[1] and liver involvement.[18] Documented conditions include nephritic and nephrotic syndrome, hypothyroidism due to interstitial fibrosis, periportal fibrosis, interstitial pneumopathy, dermatitis/atopic eczema,[7] autoimmune hemolytic anemia,[17] autoimmune hepatitis, chronic pancreatitis,[18] and rheumatoid arthritis.[19] There has also been multiple reports of thymoma presenting with autoimmune enteropathy.[20] Those with autoimmune enteropathy may have systematic autoimmune diseases such as APECED or IPEX.[1] Up to 83% of those with autoimmune enteropathy have one or more autoimmune disorders. These systematic manifestations may occur as a part of a syndrome or in isolation.[3]
Causes
While the degree of gastrointestinal involvement differs in syndromic forms, autoimmune enteropathy frequently occurs in conjunction with a systemic syndrome.[5] The two primary syndromes are autoimmune polyendocrine syndrome type 1 (APS-1) and immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome.[21] With 1 in 1.6 million cases of IPEX and 1 in 80,000–130,000 cases of APS-1, both conditions are extremely rare.[22] Certain populations, such as Finnish and Iranian Jews, have higher rates of APS-1 prevalence.[23]
IPEX mainly affects males and is an X-linked recessive condition caused by mutations causing loss of function in the FOXP3 gene found on the X chromosome.[24] The Scurfin protein, which regulates the development of CD4+CD25+ regulatory T cells, is encoded by FOXP3.[25] DNA-binding domain defects in IPEX are caused by mutations in the FOXP3 gene.[26] This disrupts regulatory T cells' regular function, triggering aberrant immune reactions that cause autoimmune symptoms like enteropathy.[27] As of 2018 over 70 mutations of the FOXP3 gene have been identified.[28] The relationship between the phenotypic presentation and specific genotypes is unclear.[29] Clinical manifestations of IPEX syndrome include eczema, endocrine disorders, and autoimmune enteropathy.[27] The most prevalent endocrine disorder is type one diabetes however, adrenal insufficiency and thyroiditis are also common.[30] After one month of age, the most frequent gastrointestinal symptoms are diarrhea as well as failure to thrive.[31] Other clinical features include alopecia, nephropathy, and autoimmune hemolytic anemia.[32]
Patients are classified as "IPEX-like" if they exhibit IPEX-like characteristics but lack the FOXP3 mutation. IPEX-like disorders can have a range of mutations in CD25, CTLA-4, STAT5B, ITCH, LRBA, and STAT1.[30] These patients present with similar clinical manifestations to those observed in IPEX, and numerous mutations lead to abnormalities in the production or function of regulatory T cells.[33]
CD25 deficiency is due to mutations in the IL2Rα gene and is inherited in an autosomal recessive pattern. As a result, the IL-2 receptor is expressed abnormally.[34] Along with autoimmune enteropathy, patients often have other complications such as cytomegalovirus pneumonitis.[35]
LRBA deficiency is caused by mutations to the LRBA gene, which serves an immunomodulatory function on CTLA4. As an immune system checkpoint, CTLA-4 prevents the growth and activation of self-reactive T cells and promotes peripheral tolerance.[36] LRBA deficiency is inherited autosomal recessively and manifests as lymphoproliferation, enteropathy, recurrent infections, and other features of immune dysregulation.[37] LRBA deficiency is most likely due to B cell activation. Laboratory studies often show hypogammaglobulinemia. Gastrointestinal and pulmonary involvement are common.[38]
Both LRBA deficiency and CTLA-4 haploinsufficiency have a significant overlap in that they can cause recurrent infections and immune dysregulation. Because individuals with LRBA deficiency usually have lower levels of CTLA-4 than those with CTLA-4 haploinsufficiency, the condition frequently presents earlier in life.[39] CTLA-4 haploinsufficiency results in abnormal regulatory T cell function and T cell proliferation, which leads to immunodeficiency and autoimmunity traits.[40] Those with CTLA-4 haploinsufficiency with autoimmune infiltration present with frequent infections, cytopenia, and lymphoproliferation.[41] Additionally, patients can present with symptoms associated with autoimmune enteropathy and autoimmune lymphoproliferative syndrome.[42]
"IPEX-like disorders" can also be caused by mutations in the STAT genes, particularly STAT5B and STAT1. Because STAT5B is involved in the formation of regulatory T cells, mutations in this gene are likely to cause abnormal T lymphocyte proliferation.[43] Those with STAT5B mutations present with pulmonary disease, immunodeficiency, and growth failure.[44]
ITCH mutations can lead to low immune tolerance and present as developmental delay, chronic lung disease, and failure to thrive.[45]
Autoimmune polyendocrine syndrome type 1 (APS-1) is caused by mutations in the AIRE gene located on chromosome 21 and is an autosomal recessive condition. More than 100 mutations of AIRE gene have been recorded.[46] The classic triad of symptoms in APS-1 is hypoparathyroidism, adrenal insufficiency, and mucocutaneous candidiasis. The majority of APS-1 patients start showing symptoms early in childhood and gradually get worse as they get older. Usually, the first symptom to appear is candidiasis, which usually affects the nails or oral cavity. Adrenal insufficiency and hypoparathyroidism often follow, usually manifesting between the ages of 5 and 15.[23] Other symptoms of APS-1 include thyroid disease, autoimmune hepatitis, type one diabetes, keratitis, alopecia, gastritis, hypogonadism, and vitiligo.[47]
Diagnosis
Autoimmune enteropathy is diagnosed by a combination of histological changes on small bowel biopsy, serologic testing, and clinical signs and symptoms. Laboratory findings such as intestinal epithelial autoantibodies aid in confirming the diagnosis.[5]
The original diagnostic criteria of autoimmune enteropathy included intractable diarrhea that showed no improvement with diet modifications, no known immunodeficiency, and villous atrophy of the small intestine.[48] More recent studies of adults with autoimmune enteropathy expanded the criteria to include prolonged diarrhea (lasting longer than six weeks) accompanied by malabsorption, diminished intraepithelial lymphocytosis, deep crypt lymphocytosis, increased crypt apoptotic bodies, and the exclusion of other causes of villous atrophy; each of the symptoms listed above is required for a diagnosis.[12]
The most common site of autoimmune enteropathy is the duodenum however, autoimmune enteropathy may also affect other parts of the gastrointestinal tract. Visually, endoscopy may be normal, abnormal findings include ulcerations and mucosal hyperemia.[49] Histopathological features include small bowel villous changes such as atrophy and blunting, typically prominent in the proximal bowel.[12] Occasionally crypt abscesses are also seen.[50] The crypt epithelium may contain apoptotic bodies and lymphocytic infiltration, with comparatively little surface lymphocytosis (less than 40 lymphocytes per 100 epithelial cells).[51] Furthermore, the intestinal mucosa contains CD4-CD8 T lymphocytes and macrophages; goblet and Paneth cells may not be present.[52] On crypt enterocytes, there is an increase in HLA class II molecular expression.[1]
With the possible exception of prominent mesenteric lymph nodes, which may be detected in up to 40% of cases, Abdominal Imaging is typically unremarkable.[9] An important diagnostic technique for determining the autoimmune enteropathy diagnosis is wireless capsule endoscopy.[53] In about 47% of patients with autoimmune enteropathy, capsule endoscopy typically detects small intestinal abnormalities, mostly in the form of scalloping, mosaic pattern, mucosal fissuring, or sporadically mucosal edema and aphthous ulceration, which are primarily limited to the proximal small bowel.[12]
Other laboratory abnormalities such as elevated hepatic transaminases, 67% of patients, mild immune-globulin deficiencies of IgG, IgM, or IgA in 33%, and vitamin deficiencies in 90%.[12]
Antibodies such as anti-smooth muscle antibodies, anti-liver/kidney microsomal antibodies, and the antinuclear antibody are sometimes positive however, this may be due to comorbid autoimmune disorders as appose to autoimmune enteropathy.[54] The presence of anti-goblet or anti-enterocyte cell antibodies raises the likelihood of autoimmune enteropathy, but are sometimes not present and are therefore not requires to establish diagnosis.[55]
Differential diagnosis
Autoimmune enteropathy and CVID share a number of characteristics, which can complicate diagnosis.[5] Features found in both disorders include crypt destruction and villous atrophy.[56] When doing a biopsy, plasma cell loss can aid in differentiating between the two conditions because, although plasma cells may not be present in CVID, autoimmune enteropathy is frequently characterized by a high number of plasma cells.[6]
Based on histologic findings the differential diagnosis of pediatric autoimmune enteropathy is graft-versus-host disease, Crohn's disease, and food sensitivity enteropathies such as celiac disease and lactose intolerance.[6]
Lactose intolerance should be excluded in infants with intractable diarrhea. Like autoimmune enteropathy, lactose intolerance can affect the whole GI tract with primary findings in the small bowel. Biopsy reveals prominent mononuclear cell infiltrate of the lamina propria, reveal flattened villi, and edema. Unlike autoimmune enteropathy, lactose intolerance is often characterized by absent crypt apoptosis and increased eosinophils.[6]
There are similarities between Crohn's disease and autoimmune enteropathy concerning their clinical and pathological presentations. However, rather than a lymphoplasmacytic infiltrate, the mucosal injury associated with Crohn's disease is more frequently accompanied by acute inflammation. Granulomas additionally support the diagnosis of Crohn's disease.[6]
There is no difference between autoimmune enteropathy and graft-versus-host disease when it comes to apoptosis. Correlation with the patient's clinical history is crucial in this situation.[6]
Treatment
Many patients with autoimmune enteropathy develop malnutrition. Oral nutritional supplements may help manage malnutrition however parenteral nutrition is often required.[7] Corticosteroids such as prednisone and budesonide are often the first line of treatment. In those who do not respond to corticosteroids immunosuppressive drugs such as infliximab,[57] tacrolimus,[58] 6-mercaptopurine,[59] sirolimus,[60] azathioprine, mycophenolate mofetil, rituximab, and cyclosporine have been used.[9] These medications have also been used alongside corticosteroids as maintenance therapy. These medications often have adverse side effects and don't always help maintain remission.[7]
Mesenchymal stem cell therapy has also been used to treat autoimmune enteropathy and has been shown to be curative.[3]
Outlook
Without treatment mortality rates of autoimmune enteropathy are as high as 30%.[8] Many factors such as the need for parenteral nutrition,[61] age of presentation, and the severity of symptoms can impact long-term outcomes.[3] No one treatment has been proven successful in all cases and relapses are common.[4]
Epidemiology
Autoimmune enteropathy is estimated to occur in less than 1 in 100,000 infants.[1]
There has been an increasing amount of adult-onset autoimmune enteropathy. The median age of diagnosis is 55 years old in adults with autoimmune enteropathy. 87% of these patients were white and there seems to be an equal distribution of females and males.[12]
See also
References
- 1 2 3 4 5 6 7 8 Montalto, Massimo; D'Onofrio, Ferruccio; Santoro, Luca; Gallo, Antonella; Gasbarrini, Antonio; Gasbarrini, Giovanni (2009). "Autoimmune enteropathy in children and adults". Scandinavian Journal of Gastroenterology. Informa UK Limited. 44 (9): 1029–1036. doi:10.1080/00365520902783691. ISSN 0036-5521. Retrieved December 1, 2023.
- 1 2 Ruemmele, Frank M.; Brousse, Nicole; Goulet, Olivier (2004). "Autoimmune enteropathy: molecular concepts". Current Opinion in Gastroenterology. Ovid Technologies (Wolters Kluwer Health). 20 (6): 587–591. doi:10.1097/00001574-200411000-00014. ISSN 0267-1379. Retrieved December 1, 2023.
- 1 2 3 4 5 Ahmed, Zunirah; Imdad, Aamer; Connelly, James A.; Acra, Sari (November 10, 2018). "Autoimmune Enteropathy: An Updated Review with Special Focus on Stem Cell Transplant Therapy". Digestive Diseases and Sciences. Springer Science and Business Media LLC. 64 (3): 643–654. doi:10.1007/s10620-018-5364-1. ISSN 0163-2116. PMC 8260026. Retrieved December 1, 2023.
- 1 2 Umetsu, Sarah E.; Brown, Ian; Langner, Cord; Lauwers, Gregory Y. (October 11, 2017). "Autoimmune enteropathies". Virchows Archiv. Springer Science and Business Media LLC. 472 (1): 55–66. doi:10.1007/s00428-017-2243-7. ISSN 0945-6317. Retrieved December 1, 2023.
- 1 2 3 4 5 6 Chen, Charles B.; Tahboub, Farah; Plesec, Thomas; Kay, Marsha; Radhakrishnan, Kadakkal (August 24, 2020). "A Review of Autoimmune Enteropathy and Its Associated Syndromes". Digestive Diseases and Sciences. Springer Science and Business Media LLC. 65 (11): 3079–3090. doi:10.1007/s10620-020-06540-8. ISSN 0163-2116. Retrieved December 1, 2023.
- 1 2 3 4 5 6 Singhi, Aatur D; Goyal, Alka; Davison, Jon M; Regueiro, Miguel D; Roche, Robyn L; Ranganathan, Sarangarajan (2014). "Pediatric autoimmune enteropathy: an entity frequently associated with immunodeficiency disorders". Modern Pathology. Elsevier BV. 27 (4): 543–553. doi:10.1038/modpathol.2013.150. ISSN 0893-3952. Retrieved December 1, 2023.
- 1 2 3 4 5 Blanco Quirós, A.; Arranz Sanz, E.; Bernardo Ordiz, D.; Garrote Adrados, J.A. (2009). "From autoimmune enteropathy to the IPEX (immune dysfunction, polyendocrinopathy, enteropathy, X-linked) syndrome". Allergologia et Immunopathologia. Codon Publications. 37 (4): 208–215. doi:10.1016/j.aller.2009.04.002. ISSN 0301-0546. Retrieved December 1, 2023.
- 1 2 Seidman, Ernest G.; Lacaille, Florence; Russo, Pierre; Galeano, Narmer; Murphy, Gerard; Roy, Claude C. (1990). "Successful treatment of autoimmune enteropathy with cyclosporine". The Journal of Pediatrics. Elsevier BV. 117 (6): 929–932. doi:10.1016/s0022-3476(05)80140-4. ISSN 0022-3476. Retrieved December 1, 2023.
- 1 2 3 Biagi, F; Corazza, GR (1997). "Autoimmune enteropathy in adults". The Lancet. Elsevier BV. 350 (9082): 960. doi:10.1016/s0140-6736(05)63302-8. ISSN 0140-6736.
- ↑ WALKERSMITH, J (1982). "AUTOANTIBODIES AGAINST GUT EPITHELIUM IN CHILD WITH SMALL-INTESTINAL ENTEROPATHY". The Lancet. Elsevier BV. 319 (8271): 566–567. doi:10.1016/s0140-6736(82)92076-1. ISSN 0140-6736. Retrieved December 1, 2023.
- ↑ Sakaguchi, Shimon (March 22, 2005). "Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self". Nature Immunology. Springer Science and Business Media LLC. 6 (4): 345–352. doi:10.1038/ni1178. ISSN 1529-2908. Retrieved December 1, 2023.
- 1 2 3 4 5 6 Akram, Salma; Murray, Joseph A.; Pardi, Darrell S.; Alexander, Glenn L.; Schaffner, John A.; Russo, Pierre A.; Abraham, Susan C. (2007). "Adult Autoimmune Enteropathy: Mayo Clinic Rochester Experience". Clinical Gastroenterology and Hepatology. Elsevier BV. 5 (11): 1282–1290. doi:10.1016/j.cgh.2007.05.013. ISSN 1542-3565. PMC 2128725. Retrieved December 1, 2023.
- ↑ Hill, S M; Milla, P J; Bottazzo, G F; Mirakian, R (January 1, 1991). "Autoimmune enteropathy and colitis: is there a generalised autoimmune gut disorder?". Gut. BMJ. 32 (1): 36–42. doi:10.1136/gut.32.1.36. ISSN 0017-5749. PMC 1379210. Retrieved December 1, 2023.
- ↑ Cuenod, Bana; Brousse, Nicole; Goulet, Olivier; De Potter, Sophie; Mougenot, Jean-François; Ricour, Claude; Guy-Grand, Delphine; Cerf-Bensussan, Nadine (1990). "Classification of intractable diarrhea in infancy using clinical and immunohistological criteria". Gastroenterology. Elsevier BV. 99 (4): 1037–1043. doi:10.1016/0016-5085(90)90624-a. ISSN 0016-5085. Retrieved December 1, 2023.
- ↑ Shihaz, Ambalathu Veettil Hussain; Paul, Jayanta (October 19, 2020). "Autoimmune enteropathy in <scp>adults</scp>". Advances in Digestive Medicine. Wiley. 9 (2): 75–81. doi:10.1002/aid2.13234. ISSN 2351-9797. Retrieved December 1, 2023.
- ↑ Carroccio, Antonio; Volta, Umberto; Di Prima, Lidia; Petrolini, Nunzio; Florena, Ada Maria; Averna, Maurizio R.; Montalto, Giuseppe; Notarbartolo, Alberto (2003). "CASE REPORT: Autoimmune Enteropathy and Colitis in an Adult Patient". Digestive Diseases and Sciences. Springer Science and Business Media LLC. 48 (8): 1600–1606. doi:10.1023/a:1024705032326. ISSN 0163-2116. Retrieved December 1, 2023.
- 1 2 Russo, Pierre A.; Brochu, Pierre; Seidman, Ernest G.; Roy, Claude C. (1999). "Autoimmune Enteropathy". Pediatric and Developmental Pathology. SAGE Publications. 2 (1): 65–71. doi:10.1007/s100249900092. ISSN 1093-5266. Retrieved December 1, 2023.
- 1 2 Lachaux, A (1996). "L'entéropathie auto-immune". Archives de Pédiatrie (in French). Elsevier BV. 3 (3): 261–266. doi:10.1016/0929-693x(96)81306-6. ISSN 0929-693X. Retrieved December 1, 2023.
- ↑ VOLTA, U; DEANGELIS, G; GRANITO, A; PETROLINI, N; FIORINI, E; GUIDI, M; MURATORI, P; BIANCHI, F (2006). "Autoimmune enteropathy and rheumatoid arthritis: A new association in the field of autoimmunity". Digestive and Liver Disease. Elsevier BV. 38 (12): 926–929. doi:10.1016/j.dld.2006.02.003. ISSN 1590-8658. Retrieved December 1, 2023.
- ↑ Mais, Daniel D.; Mulhall, Brian P.; Adolphson, Kimberly R.; Yamamoto, Kazunori (December 1, 1999). "Thymoma-Associated Autoimmune Enteropathy: A Report of Two Cases". American Journal of Clinical Pathology. Oxford University Press (OUP). 112 (6): 810–815. doi:10.1093/ajcp/112.6.810. ISSN 0002-9173.
- ↑ Barzaghi, Federica; Passerini, Laura; Bacchetta, Rosa (2012). "Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked Syndrome: A Paradigm of Immunodeficiency with Autoimmunity". Frontiers in Immunology. Frontiers Media SA. 3. doi:10.3389/fimmu.2012.00211. ISSN 1664-3224. PMC 3459184. Retrieved December 1, 2023.
- ↑ Kadakia, Sejal; Farnaes, Lauge; Dimmock, David; Chowdhury, Shimul; Ding, Yan; Anderson, Eric J.; Kingsmore, Stephen; Newfield, Ron S. (September 27, 2019). "Diagnosis and treatment of a boy with IPEX syndrome presenting with diabetes in early infancy". Clinical Case Reports. Wiley. 7 (11): 2123–2127. doi:10.1002/ccr3.2438. ISSN 2050-0904. PMC 6878034.
- 1 2 Husebye, E. S.; Perheentupa, J.; Rautemaa, R.; Kämpe, O. (April 6, 2009). "Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I". Journal of Internal Medicine. Wiley. 265 (5): 514–529. doi:10.1111/j.1365-2796.2009.02090.x. ISSN 0954-6820. Retrieved December 1, 2023.
- ↑ van der Vliet, Hans J. J.; Nieuwenhuis, Edward E. (2007). "IPEX as a Result of Mutations in FOXP3". Clinical and Developmental Immunology. Hindawi Limited. 2007: 1–5. doi:10.1155/2007/89017. ISSN 1740-2522. PMC 2248278. Retrieved December 1, 2023.
- ↑ Yagi, Haruhiko; Nomura, Takashi; Nakamura, Kyoko; Yamazaki, Sayuri; Kitawaki, Toshio; Hori, Shohei; Maeda, Michiyuki; Onodera, Masafumi; Uchiyama, Takashi; Fujii, Shingo; Sakaguchi, Shimon (October 4, 2004). "Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells". International Immunology. Oxford University Press (OUP). 16 (11): 1643–1656. doi:10.1093/intimm/dxh165. hdl:2433/144729. ISSN 1460-2377. Retrieved December 1, 2023.
- ↑ Ben-Skowronek, Iwona (February 24, 2021). "IPEX Syndrome: Genetics and Treatment Options". Genes. MDPI AG. 12 (3): 323. doi:10.3390/genes12030323. ISSN 2073-4425. PMC 7995986. Retrieved December 1, 2023.
- 1 2 Murguia-Favela, Luis; Hong-Diep Kim, Vy; Upton, Julia; Thorner, Paul; Reid, Brenda; Atkinson, Adelle; Grunebaum, Eyal (March 1, 2015). "IPEX syndrome caused by a novel mutation in FOXP3 gene can be cured by bone marrow transplantation from an unrelated donor after myeloablative conditioning". LymphoSign Journal. LymphoSign Journal Limited Partnership. 2 (1): 31–38. doi:10.14785/lpsn-2014-0016. ISSN 2292-5937. Retrieved December 1, 2023.
- ↑ Bacchetta, Rosa; Barzaghi, Federica; Roncarolo, Maria‐Grazia (February 25, 2016). "From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation". Annals of the New York Academy of Sciences. Wiley. 1417 (1): 5–22. doi:10.1111/nyas.13011. ISSN 0077-8923. Retrieved December 1, 2023.
- ↑ Seidel, Markus G.; Boztug, Kaan; Haas, Oskar A. (December 10, 2015). "Immune Dysregulation Syndromes (IPEX, CD27 Deficiency, and Others): Always Doomed from the Start?". Journal of Clinical Immunology. Springer Science and Business Media LLC. 36 (1): 6–7. doi:10.1007/s10875-015-0218-5. ISSN 0271-9142. Retrieved December 1, 2023.
- 1 2 Gambineri, Eleonora; Ciullini Mannurita, Sara; Hagin, David; Vignoli, Marina; Anover-Sombke, Stephanie; DeBoer, Stacey; Segundo, Gesmar R. S.; Allenspach, Eric J.; Favre, Claudio; Ochs, Hans D.; Torgerson, Troy R. (November 1, 2018). "Clinical, Immunological, and Molecular Heterogeneity of 173 Patients With the Phenotype of Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked (IPEX) Syndrome". Frontiers in Immunology. Frontiers Media SA. 9. doi:10.3389/fimmu.2018.02411. ISSN 1664-3224. PMC 6223101. Retrieved December 1, 2023.
- ↑ Barzaghi, Federica; Amaya Hernandez, Laura Cristina; Neven, Benedicte; Ricci, Silvia; Kucuk, Zeynep Yesim; Bleesing, Jack J.; Nademi, Zohreh; Slatter, Mary Anne; Ulloa, Erlinda Rose; Shcherbina, Anna; Roppelt, Anna; Worth, Austen; Silva, Juliana; Aiuti, Alessandro; Murguia-Favela, Luis; Speckmann, Carsten; Carneiro-Sampaio, Magda; Fernandes, Juliana Folloni; Baris, Safa; Ozen, Ahmet; Karakoc-Aydiner, Elif; Kiykim, Ayca; Schulz, Ansgar; Steinmann, Sandra; Notarangelo, Lucia Dora; Gambineri, Eleonora; Lionetti, Paolo; Shearer, William Thomas; Forbes, Lisa R.; Martinez, Caridad; Moshous, Despina; Blanche, Stephane; Fisher, Alain; Ruemmele, Frank M.; Tissandier, Come; Ouachee-Chardin, Marie; Rieux-Laucat, Frédéric; Cavazzana, Marina; Qasim, Waseem; Lucarelli, Barbarella; Albert, Michael H.; Kobayashi, Ichiro; Alonso, Laura; Diaz De Heredia, Cristina; Kanegane, Hirokazu; Lawitschka, Anita; Seo, Jong Jin; Gonzalez-Vicent, Marta; Diaz, Miguel Angel; Goyal, Rakesh Kumar; Sauer, Martin G.; Yesilipek, Akif; Kim, Minsoo; Yilmaz-Demirdag, Yesim; Bhatia, Monica; Khlevner, Julie; Richmond Padilla, Erick J.; Martino, Silvana; Montin, Davide; Neth, Olaf; Molinos-Quintana, Agueda; Valverde-Fernandez, Justo; Broides, Arnon; Pinsk, Vered; Ballauf, Antje; Haerynck, Filomeen; Bordon, Victoria; Dhooge, Catharina; Garcia-Lloret, Maria Laura; Bredius, Robbert G.; Kałwak, Krzysztof; Haddad, Elie; Seidel, Markus Gerhard; Duckers, Gregor; Pai, Sung-Yun; Dvorak, Christopher C.; Ehl, Stephan; Locatelli, Franco; Goldman, Frederick; Gennery, Andrew Richard; Cowan, Mort J.; Roncarolo, Maria-Grazia; Bacchetta, Rosa (2018). "Long-term follow-up of IPEX syndrome patients after different therapeutic strategies: An international multicenter retrospective study". Journal of Allergy and Clinical Immunology. Elsevier BV. 141 (3): 1036–1049.e5. doi:10.1016/j.jaci.2017.10.041. hdl:1854/LU-8559319. ISSN 0091-6749. Retrieved December 1, 2023.
- ↑ Barker, Jennifer M.; Anderson, Mark S.; Gottlieb, Peter A. (2016). "The Immunoendocrinopathy Syndromes". Williams Textbook of Endocrinology. Elsevier. p. 1762–1775. doi:10.1016/b978-0-323-29738-7.00040-x. Retrieved December 1, 2023.
- ↑ Charbonnier, Louis-Marie; Janssen, Erin; Chou, Janet; Ohsumi, Toshiro K.; Keles, Sevgi; Hsu, Joyce T.; Massaad, Michel J.; Garcia-Lloret, Maria; Hanna-Wakim, Rima; Dbaibo, Ghassan; Alangari, Abdullah A.; Alsultan, Abdulrahman; Al-Zahrani, Daifulah; Geha, Raif S.; Chatila, Talal A. (2015). "Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked–like disorder caused by loss-of-function mutations in LRBA". Journal of Allergy and Clinical Immunology. Elsevier BV. 135 (1): 217–227.e9. doi:10.1016/j.jaci.2014.10.019. ISSN 0091-6749. PMC 4289093. Retrieved December 1, 2023.
- ↑ Vignoli, Marina; Ciullini Mannurita, Sara; Fioravanti, Antonella; Tumino, Manuela; Grassi, Alessia; Guariso, Graziella; Favre, Claudio; D'Elios, Mario M.; Gambineri, Eleonora (2019). "CD25 deficiency: A new conformational mutation prevents the receptor expression on cell surface". Clinical Immunology. Elsevier BV. 201: 15–19. doi:10.1016/j.clim.2019.02.003. hdl:2158/1161038. ISSN 1521-6616. Retrieved December 1, 2023.
- ↑ Tivol, Elizabeth A.; Gorski, Jack (August 15, 2002). "Re-establishing Peripheral Tolerance in the Absence of CTLA-4: Complementation by Wild-Type T Cells Points to an Indirect Role for CTLA-4". The Journal of Immunology. The American Association of Immunologists. 169 (4): 1852–1858. doi:10.4049/jimmunol.169.4.1852. ISSN 0022-1767. Retrieved December 1, 2023.
- ↑ Eren Akarcan, Sanem; Edeer Karaca, Neslihan; Aksu, Guzide; Aykut, Ayca; Yilmaz Karapinar, Deniz; Cetin, Funda; Aydinok, Yesim; Azarsiz, Elif; Gambineri, Eleonora; Cogulu, Ozgur; Ulusoy Severcan, Ezgi; Alper, Hudaver; Kutukculer, Necil (October 1, 2018). "Two male siblings with a novel LRBA mutation presenting with different findings of IPEX syndrome". JMM Case Reports. Microbiology Society. 5 (10). doi:10.1099/jmmcr.0.005167. ISSN 2053-3721. PMC 6249428. Retrieved December 1, 2023.
- ↑ Lopez-Herrera, Gabriela; Tampella, Giacomo; Pan-Hammarström, Qiang; Herholz, Peer; Trujillo-Vargas, Claudia M.; Phadwal, Kanchan; Simon, Anna Katharina; Moutschen, Michel; Etzioni, Amos; Mory, Adi; Srugo, Izhak; Melamed, Doron; Hultenby, Kjell; Liu, Chonghai; Baronio, Manuela; Vitali, Massimiliano; Philippet, Pierre; Dideberg, Vinciane; Aghamohammadi, Asghar; Rezaei, Nima; Enright, Victoria; Du, Likun; Salzer, Ulrich; Eibel, Hermann; Pfeifer, Dietmar; Veelken, Hendrik; Stauss, Hans; Lougaris, Vassilios; Plebani, Alessandro; Gertz, E. Michael; Schäffer, Alejandro A.; Hammarström, Lennart; Grimbacher, Bodo (8 June 2012). "Deleterious Mutations in LRBA Are Associated with a Syndrome of Immune Deficiency and Autoimmunity". American Journal of Human Genetics. 90 (6): 986–1001. doi:10.1016/j.ajhg.2012.04.015. ISSN 0002-9297. PMC 3370280. PMID 22608502. Retrieved December 1, 2023.
- ↑ Hou, Tie Zheng; Verma, Nisha; Wanders, Jennifer; Kennedy, Alan; Soskic, Blagoje; Janman, Daniel; Halliday, Neil; Rowshanravan, Behzad; Worth, Austen; Qasim, Waseem; Baxendale, Helen; Stauss, Hans; Seneviratne, Suranjith; Neth, Olaf; Olbrich, Peter; Hambleton, Sophie; Arkwright, Peter D.; Burns, Siobhan O.; Walker, Lucy S. K.; Sansom, David M. (March 16, 2017). "Identifying functional defects in patients with immune dysregulation due to LRBA and CTLA-4 mutations". Blood. American Society of Hematology. 129 (11): 1458–1468. doi:10.1182/blood-2016-10-745174. ISSN 0006-4971. PMC 5438243. Retrieved December 1, 2023.
- ↑ Schubert, Desirée; Bode, Claudia; Kenefeck, Rupert; Hou, Tie Zheng; Wing, James B; Kennedy, Alan; Bulashevska, Alla; Petersen, Britt-Sabina; Schäffer, Alejandro A; Grüning, Björn A; Unger, Susanne; Frede, Natalie; Baumann, Ulrich; Witte, Torsten; Schmidt, Reinhold E; Dueckers, Gregor; Niehues, Tim; Seneviratne, Suranjith; Kanariou, Maria; Speckmann, Carsten; Ehl, Stephan; Rensing-Ehl, Anne; Warnatz, Klaus; Rakhmanov, Mirzokhid; Thimme, Robert; Hasselblatt, Peter; Emmerich, Florian; Cathomen, Toni; Backofen, Rolf; Fisch, Paul; Seidl, Maximilian; May, Annette; Schmitt-Graeff, Annette; Ikemizu, Shinji; Salzer, Ulrich; Franke, Andre; Sakaguchi, Shimon; Walker, Lucy S K; Sansom, David M; Grimbacher, Bodo (October 20, 2014). "Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations". Nature Medicine. Springer Science and Business Media LLC. 20 (12): 1410–1416. doi:10.1038/nm.3746. ISSN 1078-8956. PMC 4668597. Retrieved December 1, 2023.
- ↑ Kucuk, Zeynep Yesim; Charbonnier, Louis-Marie; McMasters, Richard L.; Chatila, Talal; Bleesing, Jack J. (2017). "CTLA-4 haploinsufficiency in a patient with an autoimmune lymphoproliferative disorder". Journal of Allergy and Clinical Immunology. Elsevier BV. 140 (3): 862–864.e4. doi:10.1016/j.jaci.2017.02.032. ISSN 0091-6749. PMC 5591763. Retrieved December 1, 2023.
- ↑ Lo, Bernice; Fritz, Jill M.; Su, Helen C.; Uzel, Gulbu; Jordan, Michael B.; Lenardo, Michael J. (August 25, 2016). "CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency". Blood. American Society of Hematology. 128 (8): 1037–1042. doi:10.1182/blood-2016-04-712612. ISSN 0006-4971. PMC 5000841. Retrieved December 1, 2023.
- ↑ Cohen, Aileen C.; Nadeau, Kari C.; Tu, Wenwei; Hwa, Vivian; Dionis, Kira; Bezrodnik, Liliana; Teper, Alejandro; Gaillard, Maria; Heinrich, Juan; Krensky, Alan M.; Rosenfeld, Ron G.; Lewis, David B. (September 1, 2006). "Cutting Edge: Decreased Accumulation and Regulatory Function of CD4+CD25high T Cells in Human STAT5b Deficiency". The Journal of Immunology. The American Association of Immunologists. 177 (5): 2770–2774. doi:10.4049/jimmunol.177.5.2770. ISSN 0022-1767. Retrieved December 1, 2023.
- ↑ Bernasconi, Andrea; Marino, Roxana; Ribas, Alejandra; Rossi, Jorge; Ciaccio, Marta; Oleastro, Matías; Ornani, Alicia; Paz, Rubén; Rivarola, Marco A.; Zelazko, Marta; Belgorosky, Alicia (November 1, 2006). "Characterization of Immunodeficiency in a Patient With Growth Hormone Insensitivity Secondary to a Novel STAT5b Gene Mutation". Pediatrics. American Academy of Pediatrics (AAP). 118 (5): e1584–e1592. doi:10.1542/peds.2005-2882. ISSN 0031-4005. Retrieved December 1, 2023.
- ↑ Lohr, Naomi J.; Molleston, Jean P.; Strauss, Kevin A.; Torres-Martinez, Wilfredo; Sherman, Eric A.; Squires, Robert H.; Rider, Nicholas L.; Chikwava, Kudakwashe R.; Cummings, Oscar W.; Morton, D. Holmes; Puffenberger, Erik G. (2010). "Human ITCH E3 Ubiquitin Ligase Deficiency Causes Syndromic Multisystem Autoimmune Disease". The American Journal of Human Genetics. Elsevier BV. 86 (3): 447–453. doi:10.1016/j.ajhg.2010.01.028. ISSN 0002-9297. PMC 2833372. Retrieved December 1, 2023.
- ↑ Bruserud, Øyvind; Oftedal, Bergithe E; Wolff, Anette B; Husebye, Eystein S (2016). "AIRE-mutations and autoimmune disease". Current Opinion in Immunology. Elsevier BV. 43: 8–15. doi:10.1016/j.coi.2016.07.003. ISSN 0952-7915. Retrieved December 1, 2023.
- ↑ Kahaly, G. J.; Frommer, L. (August 17, 2017). "Polyglandular autoimmune syndromes". Journal of Endocrinological Investigation. Springer Science and Business Media LLC. 41 (1): 91–98. doi:10.1007/s40618-017-0740-9. ISSN 1720-8386. Retrieved December 1, 2023.
- ↑ Unsworth, D. J.; Walker-Smith, J. A. (1985). "Autoimmunity in Diarrhoeal Disease". Journal of Pediatric Gastroenterology and Nutrition. Ovid Technologies (Wolters Kluwer Health). 4 (3): 375–380. doi:10.1097/00005176-198506000-00009. ISSN 0277-2116. Retrieved December 1, 2023.
- ↑ Villanacci, Vincenzo; Lougaris, Vassilios; Ravelli, Alberto; Buscarini, Elisabetta; Salviato, Tiziana; Lionetti, Paolo; Salemme, Marianna; Martelossi, Stefano; De Giacomo, Costantino; Falchetti, Diego; Pelizzo, Gloria; Bassotti, Gabrio (2019). "Clinical manifestations and gastrointestinal pathology in 40 patients with autoimmune enteropathy". Clinical Immunology. Elsevier BV. 207: 10–17. doi:10.1016/j.clim.2019.07.001. ISSN 1521-6616. Retrieved December 1, 2023.
- ↑ Hartfield, Dawn; Turner, Justine; Huynh, Hien; Lidman, Per; Chaba, Todd; Lacson, Atilano (2010). "THE ROLE OF HISTOPATHOLOGY IN DIAGNOSING PROTRACTED DIARRHEA OF INFANCY". Fetal and Pediatric Pathology. Informa UK Limited. 29 (3): 144–157. doi:10.3109/15513811003777300. ISSN 1551-3815. Retrieved December 1, 2023.
- ↑ Veress, B.; Franzén, L.; Bodin, L.; Borch, K. (2004). "Duodenal intraepithelial lymphocyte‐count revisited". Scandinavian Journal of Gastroenterology. Informa UK Limited. 39 (2): 138–144. doi:10.1080/00365520310007675. ISSN 0036-5521.
- ↑ Bishu, Shrinivas; Arsenescu, Violeta; Lee, Eun Y; Vargas, H David; de Villiers, Willem JS; Arsenescu, Razvan (November 29, 2011). "Autoimmune enteropathy with a CD8+ CD7-T-cell small bowel intraepithelial lymphocytosis: case report and literature review". BMC Gastroenterology. Springer Science and Business Media LLC. 11 (1). doi:10.1186/1471-230x-11-131. ISSN 1471-230X. PMC 3287162. Retrieved December 1, 2023.
- ↑ Gram-Kampmann, Eva-Marie; Lillevang, Søren T; Detlefsen, Sönke; Laursen, Stig Borbjerg (July 6, 2015). "Wireless capsule endoscopy as a tool in diagnosing autoimmune enteropathy". BMJ Case Reports. BMJ: bcr2014207931. doi:10.1136/bcr-2014-207931. ISSN 1757-790X. PMC 4493217. Retrieved December 1, 2023.
- ↑ Masia, Ricard; Peyton, Stephen; Lauwers, Gregory Y.; Brown, Ian (2014). "Gastrointestinal Biopsy Findings of Autoimmune Enteropathy". American Journal of Surgical Pathology. Ovid Technologies (Wolters Kluwer Health). 38 (10): 1319–1329. doi:10.1097/pas.0000000000000317. ISSN 0147-5185. PMC 4162848. Retrieved December 1, 2023.
- ↑ Biagi, Federico; Bianchi, Paola I.; Trotta, Lucia; Corazza, Gino R. (2009). "Anti-Goblet Cell Antibodies for the Diagnosis of Autoimmune Enteropathy?". American Journal of Gastroenterology. Ovid Technologies (Wolters Kluwer Health). 104 (12): 3112. doi:10.1038/ajg.2009.511. ISSN 0002-9270. Retrieved December 1, 2023.
- ↑ Odetola, Oluwatobi; Ananthanarayanan, Vijayalakshmi (November 16, 2018). "Gastrointestinal Presentations of Common Variable Immunodeficiency: Hiding in Plain Sight". Archives of Pathology & Laboratory Medicine. Archives of Pathology and Laboratory Medicine. 143 (4): 525–530. doi:10.5858/arpa.2017-0372-rs. ISSN 0003-9985. Retrieved December 1, 2023.
- ↑ Elwing, Jill E.; Clouse, Ray E. (2005). "Adult-Onset Autoimmune Enteropathy in the Setting of Thymoma Successfully Treated with Infliximab". Digestive Diseases and Sciences. Springer Science and Business Media LLC. 50 (5): 928–932. doi:10.1007/s10620-005-2666-x. ISSN 0163-2116. Retrieved December 1, 2023.
- ↑ Daum, Severin; Sahin, Ergün; Jansen, Andreas; Heine, Bernhard; Riecken, Ernst-Otto; Zeitz, Martin; Schmidt, Wolfgang (2003). "Adult Autoimmune Enteropathy Treated Successfully with Tacrolimus". Digestion. S. Karger AG. 68 (2–3): 86–90. doi:10.1159/000074520. ISSN 0012-2823. Retrieved December 1, 2023.
- ↑ Miyazaki, Haruka; Hoshi, Namiko; Kohashi, Michitaka; Tokunaga, Eri; Ku, Yuna; Takenaka, Haruka; Ooi, Makoto; Yamamoto, Nobuyuki; Uemura, Suguru; Nishimura, Noriyuki; Iijima, Kazumoto; Jimbo, Keisuke; Okano, Tsubasa; Hoshino, Akihiro; Imai, Kohsuke; Kanegane, Hirokazu; Kobayashi, Ichiro; Kodama, Yuzo (January 30, 2022). "A case of autoimmune enteropathy with CTLA4 haploinsufficiency". Intestinal Research. Korean Association for the Study of Intestinal Diseases. 20 (1): 144–149. doi:10.5217/ir.2020.00041. hdl:20.500.14094/90008958. ISSN 1598-9100. Retrieved December 1, 2023.
- ↑ Azizi, G.; Abolhassani, H.; Yazdani, R.; Mohammadikhajehdehi, S.; Parvaneh, N.; Negahdari, B.; Mohammadi, J.; Aghamohammadi, A. (2017). "New therapeutic approach by sirolimus for enteropathy treatment in patients with LRBA deficiency". European Annals of Allergy and Clinical Immunology. Edra SpA. 49 (05): 235. doi:10.23822/eurannaci.1764-1489.22. ISSN 1764-1489. Retrieved December 1, 2023.
- ↑ Gambarara, M.; Bracci, F.; Diamanti, A.; Ambrosini, M.I.; Pietrobattista, A.; Knafelz, D.; Ferretti, F.; Castro, M. (2005). "Long-Term Parenteral Nutrition in Pediatric Autoimmune Enteropathies". Transplantation Proceedings. Elsevier BV. 37 (5): 2270–2271. doi:10.1016/j.transproceed.2005.03.063. ISSN 0041-1345. Retrieved December 1, 2023.
Further reading
- Gupta, Nitin K.; Yilmaz, Omer; Fisher, Mark; Yajnik, Vijay (2014). "Abatacept". Journal of Clinical Gastroenterology. Ovid Technologies (Wolters Kluwer Health). 48 (1): 55–58. doi:10.1097/mcg.0b013e3182a4e0ec. ISSN 0192-0790. PMC 4518556. Retrieved December 1, 2023.
- Moore, Lynette; Xu, Xiaoning; Davidson, Geoff; Moore, David; Carli, Mary; Ferrante, Anthony (1995). "Autoimmune enteropathy with anti-goblet cell antibodies". Human Pathology. Elsevier BV. 26 (10): 1162–1168. doi:10.1016/0046-8177(95)90283-x. ISSN 0046-8177. Retrieved December 1, 2023.