 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dihydro-1,4-benzodioxine | |
| Other names
Dihydrobenzodioxin; 1,4-Benzodioxane; Benzo-1,4-dioxane; Ethylene o-phenylene dioxide; Pyrocatechol ethylene ether | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C8H8O2 | |
| Molar mass | 136.150 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The benzodioxans are a group of isomeric chemical compounds with the molecular formula C8H8O2.[1] There are three isomers of benzodioxan, as the second atom of oxygen of the dioxane can be in a second, third or fourth position: 1,2-dioxane, 1,3-dioxane and 1,4-dioxane, which respectively give 1,2-benzodioxan, 1,3-benzodioxan and 1,4-benzodioxan.[2][3]
Derivatives
Some derivatives of 1,4-benzodioxan are used as pharmaceuticals including:[4][5][6][7]
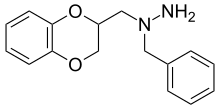
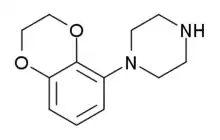
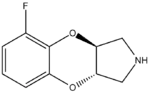
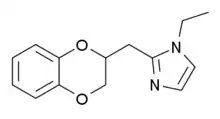
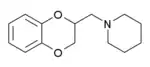
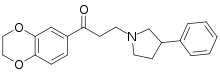
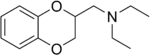 Prosympal
Prosympal
See also
References
- ↑ "1,4-Benzodioxane". PubChem. National Library of Medicine. Archived from the original on 2022-09-05. Retrieved 2022-09-05.
- ↑ "TECHNICAL FACT SHEET – 1,4-DIOXANE" (PDF). Technical Fact Sheet. 51 (6): 9. 2017. Archived (PDF) from the original on 2022-08-13. Retrieved 2022-09-05 – via United States Environmental Protection Agency.
- ↑ Jablonski, Stanley (1967). Russian drug index. Public Health Service publication. U.S. Dept. of Health, Education, and Welfare, Public Health Service: United States Department of Health.
- ↑ Sun, Juan; Cao, Ning; Zhang, Xiao-Min; Yang, Yu-Shun; Zhang, Yan-Bin; Wang, Xiao-Ming; Zhu, Hai-Liang (2011-08-15). "Oxadiazole derivatives containing 1,4-benzodioxan as potential immunosuppressive agents against RAW264.7 cells". Bioorganic & Medicinal Chemistry. 19 (16): 4895–4902. doi:10.1016/j.bmc.2011.06.061. ISSN 1464-3391. PMID 21782456.
- ↑ "1,4-benzodioxan derivatives their preparation and pharmaceutical compositions containing them - Patent IL-43272-A - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-09-05.
- ↑ Sun, Juan; Wang, Su; Sheng, Gui-Hua; Lian, Zhi-Min; Liu, Han-Yu; Zhu, Hai-Liang (2016-11-01). "Synthesis of phenylpiperazine derivatives of 1,4-benzodioxan as selective COX-2 inhibitors and anti-inflammatory agents". Bioorganic & Medicinal Chemistry. 24 (21): 5626–5632. doi:10.1016/j.bmc.2016.09.023. ISSN 1464-3391. PMID 27658794.
- ↑ Matos, M. Agostinha R.; Sousa, Clara C. S.; Morais, Victor M. F. (2008-08-28). "Experimental and computational thermochemistry of 1,4-benzodioxan and its 6-R derivatives". The Journal of Physical Chemistry A. 112 (34): 7961–7968. Bibcode:2008JPCA..112.7961M. doi:10.1021/jp803579y. ISSN 1520-5215. PMID 18683910.
External links
 Media related to Benzodioxan at Wikimedia Commons
Media related to Benzodioxan at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.