 | |
| Names | |
|---|---|
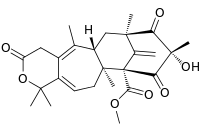
| Preferred IUPAC name
Methyl (5aS,7R,9S,11R,11aS)-9-hydroxy-1,1,5,7,9,11a-hexamethyl-14-methylidene-3,8,10-trioxo-3,4,5a,6,7,8,9,10,11a,12-decahydro-7,11-methanocycloocta[4,5]cyclohepta[1,2-c]pyran-11(1H)-carboxylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C26H32O7 | |
| Molar mass | 456.535 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Berkeleydione is a chemical compound isolated from a Penicillium species that has in vitro activity in a cancer cell line.[1] It was first discovered in fungal species which evolved to live in an acidic lake at Berkeley Pit.
Notes
- ↑ Stierle, DB; Stierle, AA; Hobbs, JD; Stokken, J; Clardy, J (2004). "Berkeleydione and berkeleytrione, new bioactive metabolites from an acid mine organism". Organic Letters. 6 (6): 1049–52. doi:10.1021/ol049852k. PMID 15012097.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.