3C5H3.png.webp)
In the area of organometallic chemistry, a bulky cyclopentadienyl ligand is jargon for a ligand of the type C
5H
5−nR−
n where R is a branched alkyl and n = 3 or 4. Representative examples are the tetraisopropyl derivative C
5HiPr−
4 and the tris(tert-butyl) derivative 1,2,4-C
5H
2tBu−
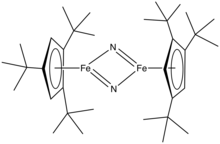
3. These ligands are so large that their complexes behave differently from the pentamethylcyclopentadienyl analogues. Because they cannot closely approach the metal, these bulky ligands stabilize high spin complexes, such as (C5H2tBu3)2Fe2I2. These large ligands stabilize highly unsaturated derivatives such as (C5H2tBu3)2Fe2N2.
Synthesis and reactions
The (tert-butyl)cyclopentadiene is prepared by alkylation of cyclopentadiene with tert-butyl bromide in the presence of sodium hydride and dibenzo-18-crown-6.[1] The intermediate in this synthesis is di-tert-butylcyclopentadiene. This compound is conveniently prepared by alkylation of cyclobutadiene with tert-butyl bromide under phase-transfer conditions.[2][1]
Illustrative of the unusual complexes made possible with these bulky ligands is molecular iron nitrido complex (tBu3C5H2)2Fe2N2.[3] In contrast to (C5Me5)2Ir2Cl4, (tBu3C5H2)IrCl2 is monomeric.[4]
References
- 1 2 Reiners, Matthias; Ehrlich, Nico; Walter, Marc D. (2018). "Synthesis of 1,3,5-Tri-tert-Butylcyclopenta-1,3-diene and Its Metal Complexes Na{1,2,4-(Me3C)3C5H2} and Mg{η5-1,2,4-(Me3C)3C5H2)2". Inorganic Syntheses. 37: 199. doi:10.1002/9781119477822.ch8.
- ↑ Hyster, Todd K. (2015). "1,3-Di-tert-butylcyclopentadiene". Encyclopedia of Reagents for Organic Synthesis: 1–2. doi:10.1002/047084289X.rn01866. ISBN 9780470842898.
- ↑ Reiners, Matthias; Maekawa, Miyuki; Daniliuc, Constantin G.; Freytag, Matthias; Jones, Peter G.; White, Peter S.; Hohenberger, Johannes; Sutter, Jörg; Meyer, Karsten; Maron, Laurent; Walter, Marc D. (2017). "Reactivity studies on [Cp′Fe(μ-I)]2: Nitrido-, sulfido- and diselenide iron complexes derived from pseudohalide activation". Chemical Science. 8 (5): 4108–4122. doi:10.1039/C7SC00570A. PMC 6099922. PMID 30155215.
- ↑ Shimogawa, Ryuichi; Takao, Toshiro; Suzuki, Hiroharu (2017). "Half-sandwich Cyclopentadienyl Iridium Dichloride Monomer Cp‡IrCl2 (Cp‡ = 1,2,4-tri-tert-butylcyclopentadienyl)". Chemistry Letters. 46 (2): 197–199. doi:10.1246/cl.160937.