| |
| Names | |
|---|---|
| IUPAC name
N2-Butyl-N1-(2-chloro-6-methylphenyl)glycinamide | |
| Systematic IUPAC name
2-(Butylamino)-N-(2-chloro-6-methylphenyl)acetamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H19ClN2O | |
| Molar mass | 254.75576 |
| Pharmacology | |
| N01BB05 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Butanilicaine is a local anesthetic. It is also known by the name Hostacaine.
Synthesis
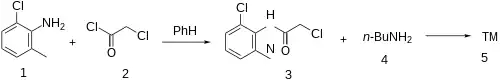
The amide formation between 2-Chloro-6-Methylaniline [87-63-8] (1) and Chloroacetyl chloride (2) gives 2-Chloro-n-(2-chloro-6-methylphenyl)acetamide [6307-67-1] (3). Alkylation with N-Butylamine (4) completed the synthesis of Butanilicaine (5).
References
- ↑ Haussler A, Ther L. [Chemical detection of hostacain]. Arzneimittelforschung. 1953;3(12):609-11.
- ↑ Epstein, Elias; Kaminsky, Daniel (1958). "N-(Substituted Aminoacyl)-chloroanilines". Journal of the American Chemical Society 80 (8): 1892–1895. doi:10.1021/ja01541a028.
- ↑ GB 726080 (1955 to Polymer Corp.)
- ↑ GB 759744 (1956 to Cilag)
- ↑ GB 782971 1957 to Hoechst AG.
- ↑ G. Ehrhart et al., U.S. Patent 2,912,460 (1959 to Hoechst).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.