 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H24N2O4 |
| Molar mass | 308.378 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
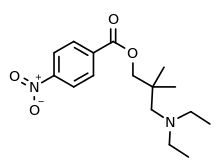
Nitracaine is a synthetic compound classified as a local anesthetic with stimulant properties. It falls into the class of drugs known as local anesthetics, and it is chemically related to cocaine. Nitracaine shares some similarities in effects with cocaine but has its own distinct pharmacological profile. The chemical structure of nitracaine consists of a benzoic acid ester with a para-substituted phenyl ring. It is formally known as 3-(diethylamino)-2,2-dimethylpropyl 4-nitrobenzoate. The presence of the nitro group (NO2) in its molecular structure contributes to its anesthetic properties. [1][2][3]
It is closely related to dimethocaine.
Legal status
Sweden's public health agency suggested classifying Nitracaine as a hazardous substance, on September 25, 2019.[4]
See also
References
- ↑ Power JD, Scott KR, Gardner EA, Curran McAteer BM, O'Brien JE, Brehon M, et al. (January 2014). "The syntheses, characterization and in vitro metabolism of nitracaine, methoxypiperamide and mephtetramine". Drug Testing and Analysis. 6 (7–8): 668–675. doi:10.1002/dta.1616. PMID 24574100.
- ↑ Acton WJ, Lanza M, Agarwal B, Jürschik S, Sulzer P, Breiev K, et al. (March 2014). "Headspace analysis of new psychoactive substances using a Selective Reagent Ionisation-Time of Flight-Mass Spectrometer". International Journal of Mass Spectrometry. 360: 28–38. Bibcode:2014IJMSp.360...28A. doi:10.1016/j.ijms.2013.12.009. PMC 4375562. PMID 25844048.
- ↑ "Nitracaine". New Synthetic Drugs Database.
- ↑ "Tretton ämnen föreslås klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. 25 September 2019.