 | |
| Names | |
|---|---|
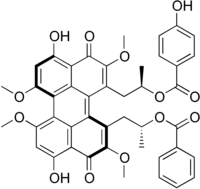
| IUPAC name
[(2R)-1-[3,10-dihydroxy-12-[(2R)-2-(4-hydroxyphenoxy)carbonyloxypropyl]-2,6,7,11-tetramethoxy-4,9-dioxoperylen-1-yl]propan-2-yl] benzoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C44H38O14 | |
| Molar mass | 790.774 g·mol−1 |
| Appearance | red to brown powder |
| log P | 7.65 |
| Acidity (pKa) | 5.46 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Calphostin C is a natural chemical compound. It is one of the calphostins, isolated from the fungus Cladosporium cladosporioides.[1][2] Calphostin C is a potent inhibitor of protein kinase C (PKC).
References
- ↑ Kobayashi, E; Ando, K; Nakano, H; Iida, T; Ohno, H; Morimoto, M; Tamaoki, T (1989). "Calphostins (UCN-1028), novel and specific inhibitors of protein kinase C. I. Fermentation, isolation, physico-chemical properties and biological activities". J. Antibiot. 42 (10): 1470–1474. doi:10.7164/antibiotics.42.1470. PMID 2478514.
- ↑ Iida, T; Kobayashi, E; Yoshida, M; Sano, H (1989). "Calphostins, novel and specific inhibitors of protein kinase C. II. Chemical structures". J. Antibiot. 42 (10): 1475–1481. doi:10.7164/antibiotics.42.1475. PMID 2478515.
External links
![]() Media related to Calphostins at Wikimedia Commons
Media related to Calphostins at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.